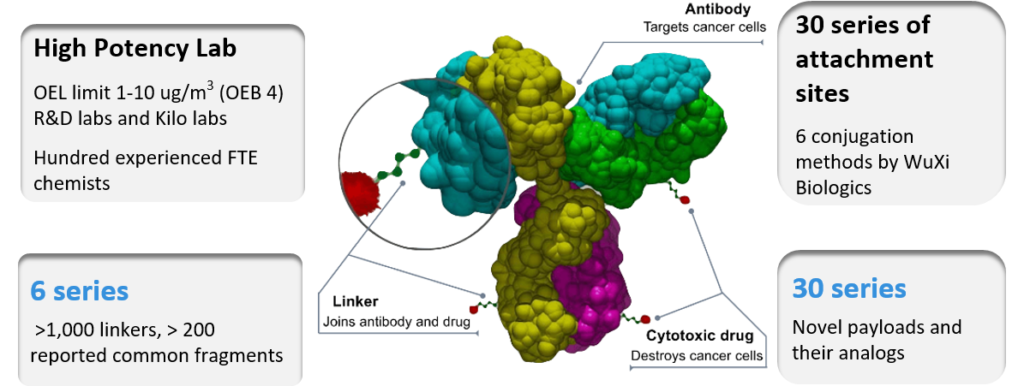
Antibody Drug Conjugate – Molecule Synthesis
We have prepared >20,000 ADC molecules. In addition, we have scaled up hundreds of grams of ADC candidate compounds for further studies.
- Considerable experience in the synthesis of novel payloads and their analogs.
- Large collection of frequently used linkers in house, including PEG linkers, alkyl linkers, click chemistry linkers, and rigid linkers.
- We have also assembled a collection of over 30 series of attachment sites.
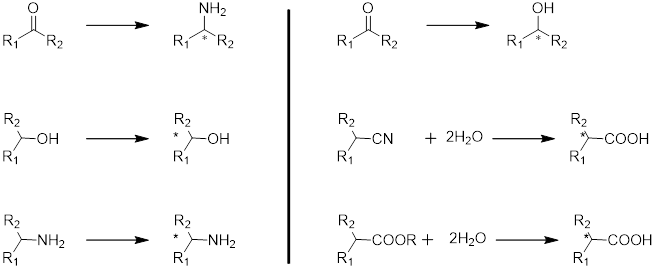
Biotransformation Platform
The Biotransformation FTE team can screen and find an optimal enzyme that shorten the synthetic sequence and aid large-scale synthesis with high selectivity, high conversion and high yield.
- Dedicated Biotransformation FTE team
- Overall Success Rate > 60%
- Cycle time < 2 days
- Enzyme used in each reaction <100 mg
Reaction Type
Enzymes (>500 in stock)
- Lipase
- Protease
- Esterase
- Nitrilase
- Transaminase
- Ketoreductases
- Phospholipase
Reactions
- Selective hydrolysis of esters
- Selective reduction of ketone
- Selective synthesis of chiral amines
- Selective synthesis of chiral alcohol
- Selective hydrolytic removal of O-acetyl groups
Catalyst Kit Screening Platform
- Auto-sampler facilitated high-throughput catalysis screening for various cross-coupling reactions
- A collection of over 100 metal catalysts and ligands
- More than 3,000 kit screenings per year in IDSU

Reaction Types:
- Buchwald reactions: N-arylation (Pd & Cu & Ni, 1°& 2°amines).
- Suzuki type reactions:
- a: sp2-sp2
- b: sp3-sp2
- c: sp3-sp3
- d: Pd Borylation of Aryl halides
- e: Ir Borylation of Arenes/heteroarene
- f: Cu Borylation of aklynes
- Negishi reactions.
- Heck and Sonogashira reactions.
- Pd catalyzed cyanation and carbonylation of Aryl Halides.
- Pd C-O & C-S Coupling with Alkyl or Aryl-O(S)H.
- Ni catalyzed reductive coupling.
And more…
Electrochemistry Platform
- Ten kinds of different electrodes (CV, Zn, Ni, RVC, C, Al, Mg, Fe, Cu, Pt) are in hand for various reaction types.
- Hundreds of grams scale could be carried out in laboratory.
- Common redox reaction and coupling, fluorination reactions could be finished successfully.
- The success rate could be up to 80% for a well documented substrate.
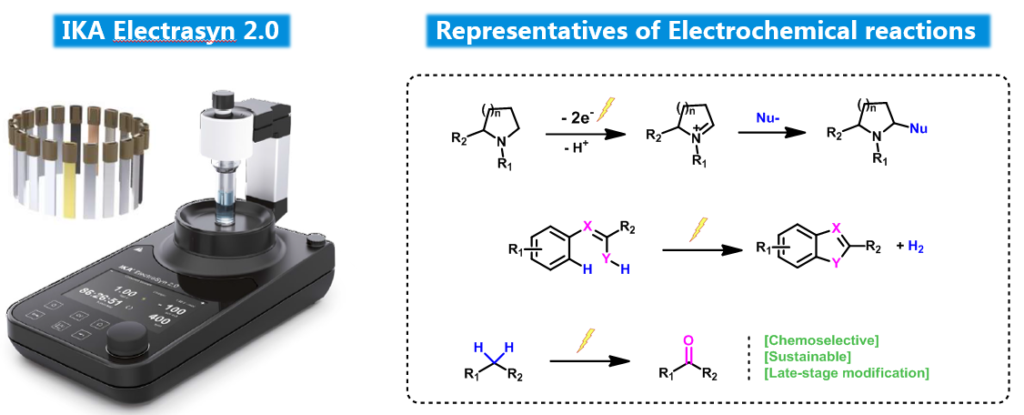
1. Redox Reaction:
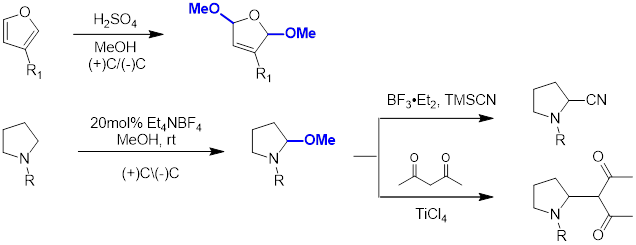
2. Fluorination Reaction:
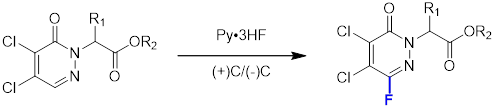
3. Coupling Reaction:
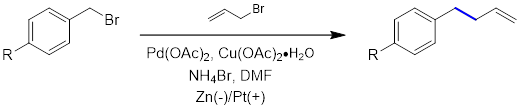
Flow Chemistry – the Better Solution for Safety/Scale-up/Efficiency
Flow chemistry is also known as continuous flow or plug flow chemistry. It involves a chemical reaction run in a continuous flow stream. The process offers potential for the efficient manufacture of chemical products.
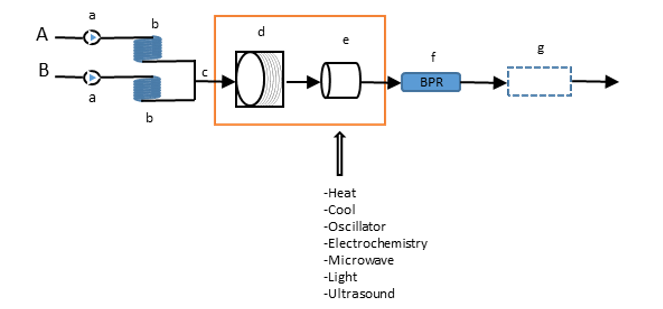
The Benefit of Flow Chemistry:
- Faster reactions
- Cleaner products
- Safer reactions
- Integrated synthesis, work-up, and analysis
- Rapid reaction optimization
- Easy scale-up
Versatile
- Easily set up and used for a wide range of reactions
- Broad temperature range (-70 ºC to 250 ºC)
- Up to 4 reactor stages
- Up to 4 independently controlled reagent pumps
- Expandable, links to 3rd party equipment
- Strong Acid compatible
- Gas / liquid
- Flow rate range of 0.05 mL/min to 10 mL/min
- Pumping pressure up to 42bar
- 10 mL reactors
Precise
• Temperature control +/- ºC across operating range
Rapid Heat-up/Cooldown
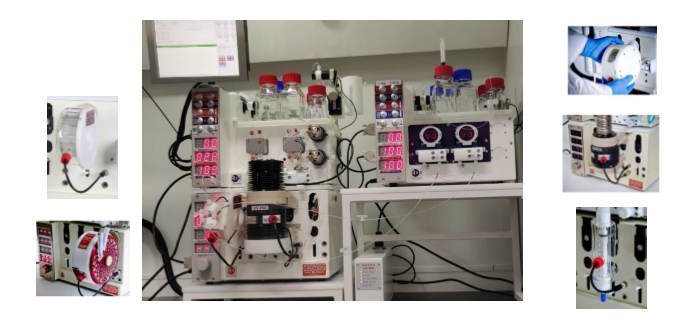
Oligonucleotide Synthesis & Conjugation
A One-stop Shop to Create Your ‘Monomer – Oligo – Conjugate’
- Focus on Discovery Scales to Support Early-stage Development | Accelerate Oligo-based Drug Discovery
- Delivering a broad spectrum of special monomers, diversified oligonucleotides, and custom-tailored solution to oligo-conjugation
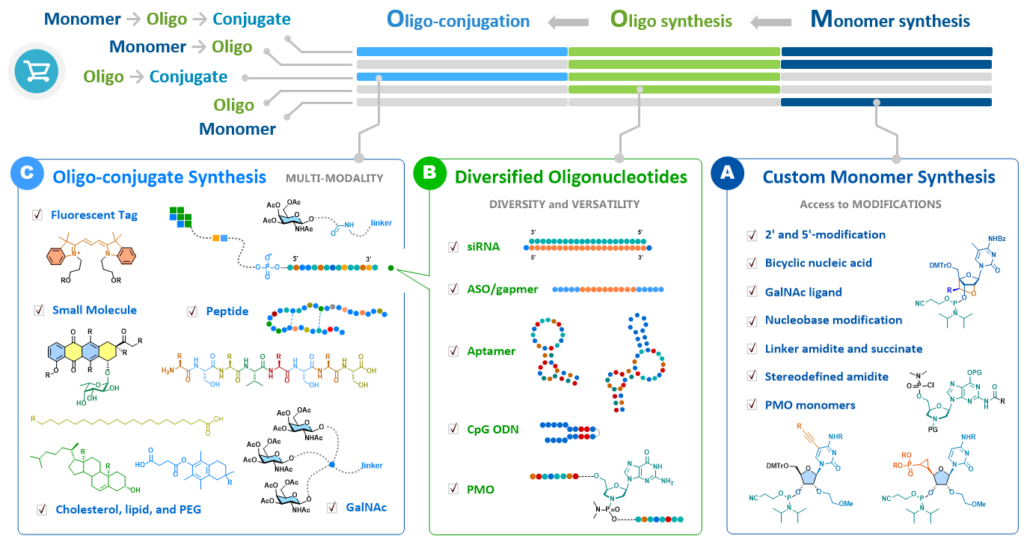
- Special monomer synthesis — bicyclic nucleic acid, stereo-defined amidite, GalNAc, PMO monomers
- Most comprehensive oligonucleotide — siRNA, antisense oligonucleotide (ASO), CpG, aptamer, PMO
- Modified oligonucleotide — various bicyclic nucleic acid, 2’-modification, inverted abasic, 5’-VP
- Linker-attached oligonucleotide — single-strand and duplex with 3’- or 5’-linker modification, including amino, azido, alkyne, tetrazine, and maleimide, toward antibody conjugation.
- Long RNA sequence — up to 100 bp, >90% purity
- Oligo-conjugation — siRNA and ASO with 3’- or 5’-conjugation, including GalNAc, Peptide, small molecule, cholesterol, lipid, PEG, fluorescent tag…
• A complete set of oligonucleotide synthesis facilities

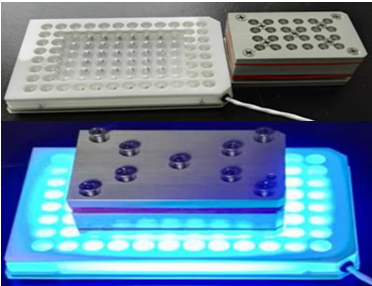
Photoredox Platform
- More than 5,000 photoreactions per year in IDSU
- Experienced in >20 various reaction types from mg to kg scale synthesis
- Optimize conditions by screening reactor and scale-up by flow reactor
MacMillan Photoreactor

Kit Screening
Flow Reactor

PROTAC®
Synthesis, Analysis, Purification and DMPK
- We have 900+ chemists with considerable experiences in the linkers, E3 ligase ligand and target protein ligand synthesis since 2016
- >60 clients/collaborations
- >50 methods of Prep-HPLC and chiral-SFC for purification
- Efficient in-vitro/in-vivo testing by WuXi DMPK
- Experienced in versatile linkers synthesis, 100+ are readily available
- Novel linker synthesis by high productivity, quality, speed, and reliability PROTAC ® Platform
- Experienced in CRBN/VHL/IAP/MDM2 and novel E3 ligase ligands synthesis, >30 CRBN/VHL ligands are readily available
- 30 series of target protein ligands coupled with various linkers

Traditional FFS
From mg to hundreds of kg
> 20,000 compounds synthesized
> 90% on-time delivery
Library
> 100 libraries finished
1~5 step parallel synthesis
2~4 weeks of turn-around time
PROTAC ® Molecular Bank
- A modular platform: More efficient PROTAC®synthesis
- Customized PROTAC®: From mg scale to 200+ kg
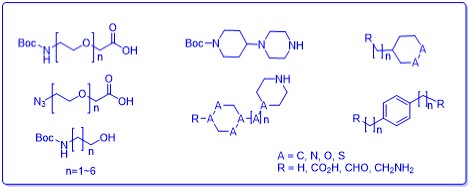
Linkers: Most frequently used linkers, e.g. PEG, alkyl, ”clickable”, and rigid linkers
- In-stock compounds: More than 350 in-stock linkers or E3 ligase ligands based on literatures
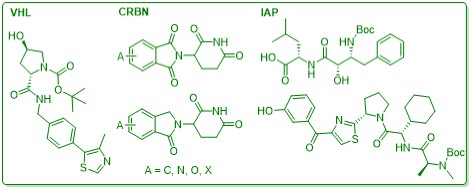
E3 ligase ligands: Common ligands are readily available, e.g. VHL, CRBN, cIAP, etc
Innovative Prospective Quantum Mechanics Analyses
Synthesis of novel molecules continues to be a serious rate limiting step in chemistry discovery. Shortening the synthetic cycle time means investing only in reactions that are more likely to work throughout the synthetic sequence and have the least number of steps. Structural diversification, changes in substitution patterns, unique heterocyclic systems can often adversely impact the crucial step(s) of established synthetic sequences or development of feasible routes. Unique arrays of functionalities in novel heterocyclic systems can lead to unexpected reactivity. Retrospective Quantum Mechanics (QM) analysis of interesting and unanticipated observations showed that we could avoid most of these dead ends and provided insight on how to successfully overcome these synthetic challenges. Real-time incorporation of QM for prospective evaluations1 has greatly enhanced our success rates, reduced cycle times, and improved overall yield of our synthetic sequences.2
References:
1Glorius, F. et al, Chem. Soc. Rev., 2020, Advance Article. Nesse, F. et al. J. Am. Chem. Soc, 2019, 141, 2814.
2 Learn more | Quantum Mechanics for Organic Chemists: An Experimentalist Approach






