A Comprehensive Virology and Viral Vector Platform
From in vitro assays setup to clinical trial support
WuXi Biology provides a comprehensive infectious disease and virology platform with integrated drug discovery services from assay design and setup, to clinical trial support. Our infectious disease team provides world class services to accelerate your drug discovery program from target validation to preclinical candidate selection and beyond.
Liver-Specific Viruses
WuXi Biology offers a broad assay platform for the discovery of agents for the treatment of liver-specific viruses infection, including services for assay establishment and validation, compound screening and characterization, and support of preclinical studies and clinical trials.
Hepatitis B virus (HBV) Platform
Chronic hepatitis B (CHB) is a severe public health burden and an unmet medical need. Current standard therapies, interferon-α and nucleot(s)ides, cannot eliminate HBV. Recently, various novel targets and approaches are being explored towards the cure of CHB. The Biology HBV team at WuXi AppTec, led by a group of experienced scientists with in-depth knowledge in anti-HBV drug discovery, has established and been providing an open access and full-range integrated HBV platform to our clients, including biochemical and cell-based assays, ex vivo PHH systems, animal models and clinical virology assays. Our HBV team is your ideal partner for discovery and development of novel anti-HBV agents.
In Vitro Assays
- HBV stable cell lines : HepG2.2.15, DE19 and DES19
- HBV DNA, cccDNA
- HBV antigens
- HBV RNAs
- Encapsidated RNA and DNA
- Capsid content
- HBV DNA constructs with transient transfection assay
- Clinical isolates/genotype A to J (~5 strains for each genotype)
- Nucleot(s)ide and capsid inhibitor resistant mutants
- Phenotyping with shuttle vectors and mutant constructs
- Fitness
- Drug sensitivity
- HepG2-NTCP cell/HBV infectious system
- Reporter cell lines (hTLR, THP1-Blue ISG, TNF-induced NF-κB-luciferase, and IFN-α/β induction SEAP)
- Production of HBV from HBV stable cell lines
- Recombinant core protein expression and purification, and capsid quenching assay
- MOA studies
Ex Vivo Primary Human Hepatocytes (PHH)
- Fresh isolated and cryopreserved PHH
- In vitro HBV infection
- In vivo HBV infection
HBV Animal Models
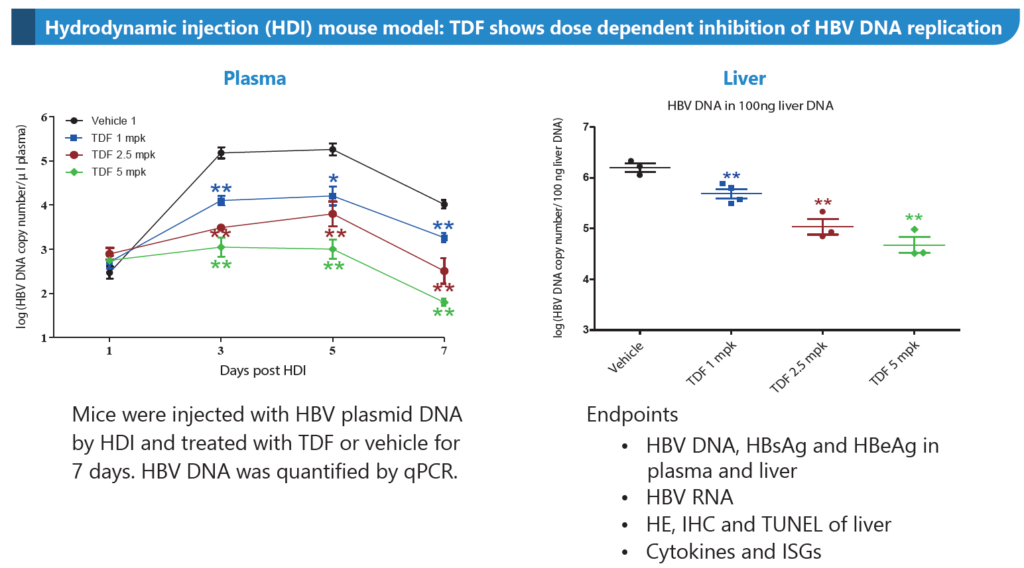
- Hydrodynamic injection (HDI) mouse model
- AAV/HBV mouse model
- Humanized FRG mouse model
- Transgenic mouse model
- WHV/woodchuck model
Clinical Virology (CAP lab)
- Viral Load (Cobas)
- Sequencing: Pol, core, full-length sequencing, sanger (clone and population), deep sequencing
- Genotyping by INNO-LiPA
- Phenotyping
- HBV emerging markers: serum HBV RNA, HBcrAg
PK/PD Study and HBV Animal Model Related Immunology Assays
- Cytokines by ELISA, Luminex and MSD
- IFN and ISG mRNA by ELISA and RT-qPCR
- Immune markers by IHC, IF and Western blot
- Splenocytes, lymphocytes from organs and PBMC by FACS, ICS and ELISPOT
Hepatitis C virus (HCV)
In Vitro Assays
- Drug Screening: stable and transient replicons, and HCVcc with different endpoints
- Profiling
- Drug resistance: cross resistance, denovo resistance selection
- Genotypic spectrum
- Drug combination
- Serum shift
- Colony formation
- Biochemistry: viral enzymes
Clinical Virology (CAP lab)
- Viral Load (Cobas)
- Genotyping
- Phenotyping (chimera, SDM, fitness,drug susceptibility)
Hepatitis D virus (HDV)
- Cell-based infectious assay
Virology Assays Supporting Human Clinical Trials
Clinical Sample Testing
- HCV
- HBV
- RSV
Automated Sample Preparation, Amplification, and Quantitation
- HCV RNA viral load
- HBV DNA viral load
Genotyping
- Taqman SNP and CNV assay
- Sanger sequencing
- SNPshot
- MSI
Gene Expression Studies
- Fresh tissues
- Whole blood
- FFPE