Immune profiling and biomarker platforms to support pre-clinical research
WuXi Biology provides discovery and clinical biomarker services for immuno-oncology research. Our technology platform includes a CAP-certified pathology lab, a flow cytometry and molecular biology lab, and GCP compliance. Our team is composed of highly-trained immunologists, board-certified pathologists, and biomarker experts.
Flow Cytometry
- >20 channel flow cytometry analysis
- Cover >60 cell marker/biomarker
- Baseline TIL (tumor-infiltrating leukocytes) data from >30 models
- Service covered TIL analysis
- Checkpoint/co-stimulatory marker analysis
- in vitro/in vivo phospho-flow
- Function analysis of immune cells
- High dimensional data mining
- View our comprehensive CD34+ Hematopoietic Stem Cell Differentiation Platform
Pathology/Multiplex IF
- FFPE preparation/IHC/IF to support pre-clinical pathology diagnosis for drug safety evaluation, efficacy evaluation, etc.
- Multiplex IF focusing on immune microenvironment analysis, including
- Immune cell infiltration analysis
- Spatial-proximity analysis
- Secretory protein source determination
- Complex tissue structure determination
- Evaluation of gene expression regulation
- Provide biomarker testing services for clinical studies, including:
- H&E and histology analysis
- FFPE sample process and slide cutting
- IHC/ICC,IF, EHC, TCT, FISH, DISH, RNAscope, and multiplex-IF
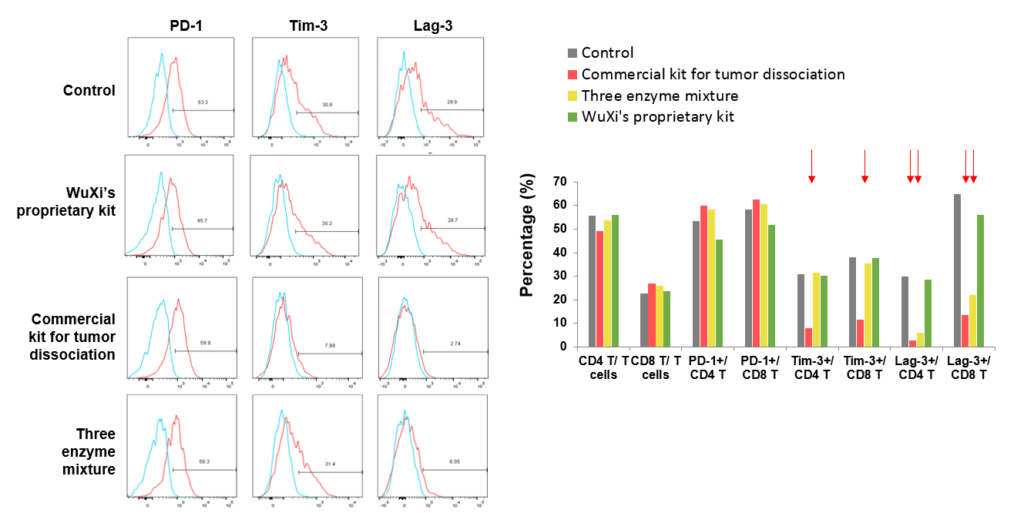
Case study: Optimization of human tumor dissociation process to eliminate hTIM-3 and hLAG-3 degradations
NanoString-based gene expression profiling
- Gene expression profiling with up to 800 genes in one panel
- Enabling GEP in difficult samples like FFPE
- Panel covering hot genes/pathways/function in immunology/oncology
Single-cell RNA sequencing platform and bioinformatics services
- One-stop service covering sample processing, library preparation, sequencing, and bioinformatics analysis.
- Enabling cell type identification and phenotyping, differential gene expression analysis, functional enrichment, and cell-cell interaction.
Molecular platform
- Quantitative polymerase chain reaction (qPCR) to support efficacy and safety evaluation (e.g. target gene copy number variation in CAR-T cells, and replication competent lentivirus (RCL) level in CAR-T cells).
- cMET exon14 skipping and EGFR mutation testing.
Related Resources View All
Poster Presentations at IMMUNOLOGY2024
Resource Type: Video
OncoWuXi Express: AAI 2024 Posters: Sneak Peek
Resource Type: Article Blog
Antibody Discovery Platform
Resource Type: Brochure
iMN041: Prodrug with a Unique Antitumor Immune Response
Resource Type: Latest Science Publication
High-Dimensional Flow Cytometry Platform
Resource Type: Latest Science Presentation
One Stop Target-to-Hit Platform: STATs
Resource Type: Brochure

