Oncolytic Viruses Platform
Oncolytic virus (OV) therapy is emerging as a promising approach for the treatment of cancers. The functional mechanisms of OV include specific lysis of tumor cells and stimulation of host immune responses. Oncolytic viruses can selectively infect and replicate in tumor cells, resulting in the lysing of these cells and release of progeny viruses which can subsequently infect neighboring tumor cells. Oncolytic viruses can also trigger host immune responses to attack tumor cells. The combination of OVs with other therapeutic agents have shown enhanced efficacy against tumors.
From Discovery to Clinical Support: Oncolytic virus engineering and production, evaluation of tumor selectivity of OVs, and evaluation of molecular variants
We provide a comprehensive, end-to-end platform including various in vitro assays and in vivo models to support discovery and development of oncolytic virus agents for the treatment of tumors.
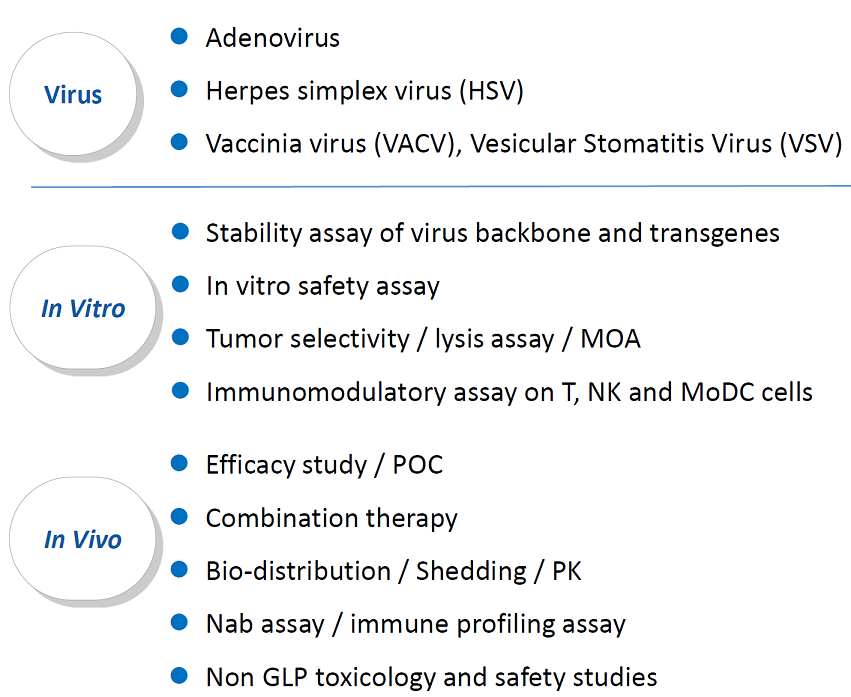
Viruses
- Adenovirus
- Herpes simplex virus
- Vaccinia virus
- Reovirus
In vitro tumor cell lysis and immune assays
- Virus specificity
- Co-culture for immune cytotoxicity analysis
- Lysis activity
- Safety assays
- Immunological assays
In vivo pharmacology
- Syngeneic and humanized models
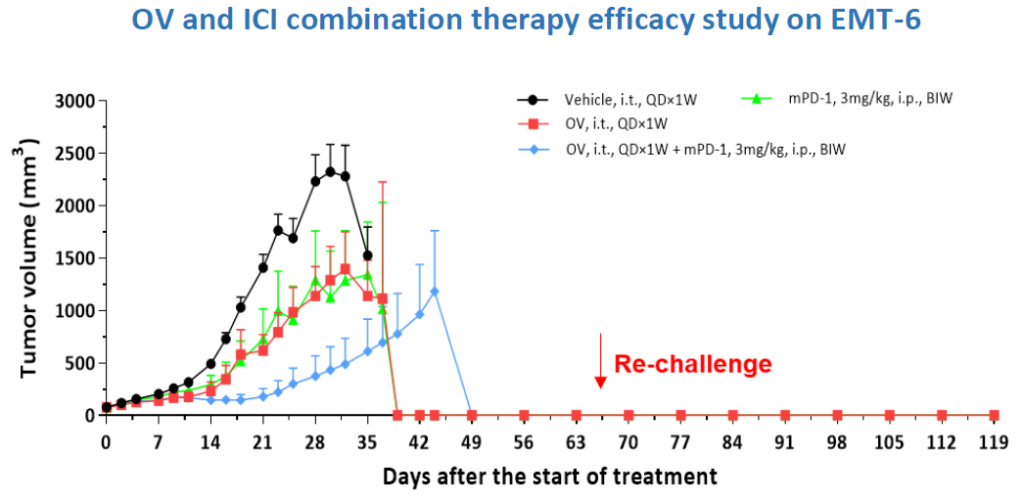
- Efficacy studies
- Combination therapy
- Biomarkers
In Vitro Assays
- Titration of virus by plaque, TCID50, and qPCR assays
- Assessment of exogenous gene expression by flow cytometry, ELISA or MSD
- Study of virus specificity with selectivity and lysis function against tumor cells
- OV-related immunogenicity includes both anti-virus and anti-transgene
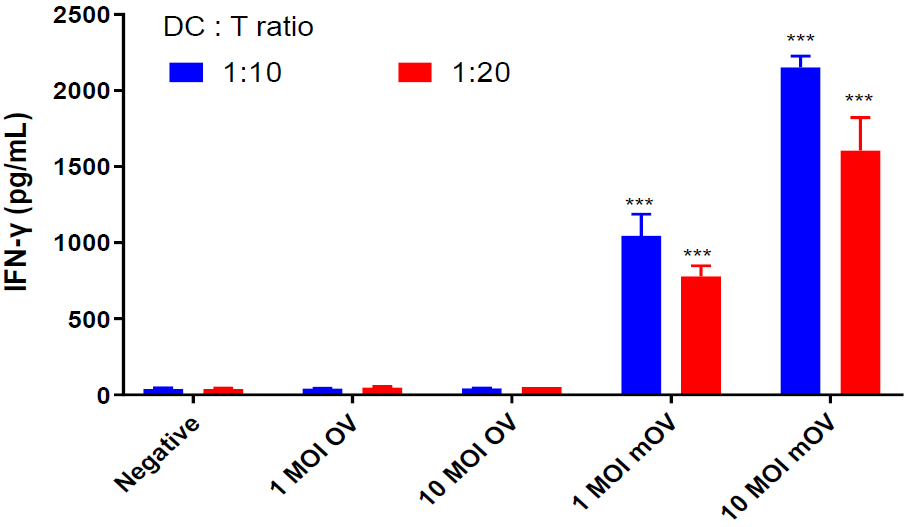
- OV-related immune cell activation and cytokine release using T cells, NK cells and monocyte-derived dendritic cells
- Assessment of biosafety
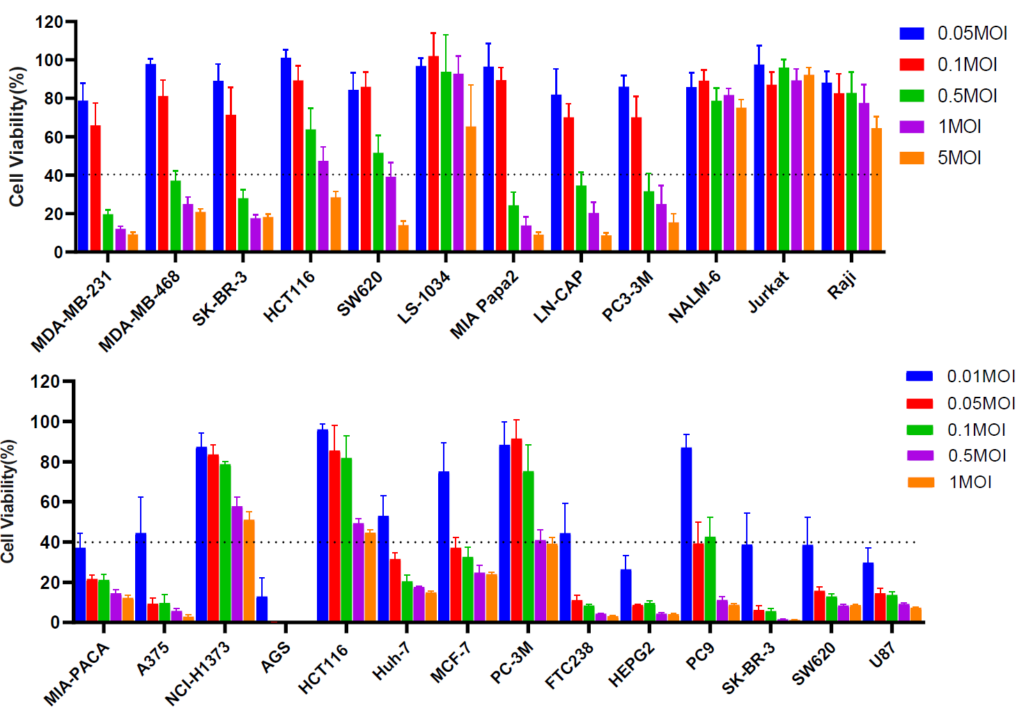
Oncology cell panel screening of VACV (top) and VSV (bottom) against different cancer cell lines at various MOI, for screening viral sensitive cell lines.
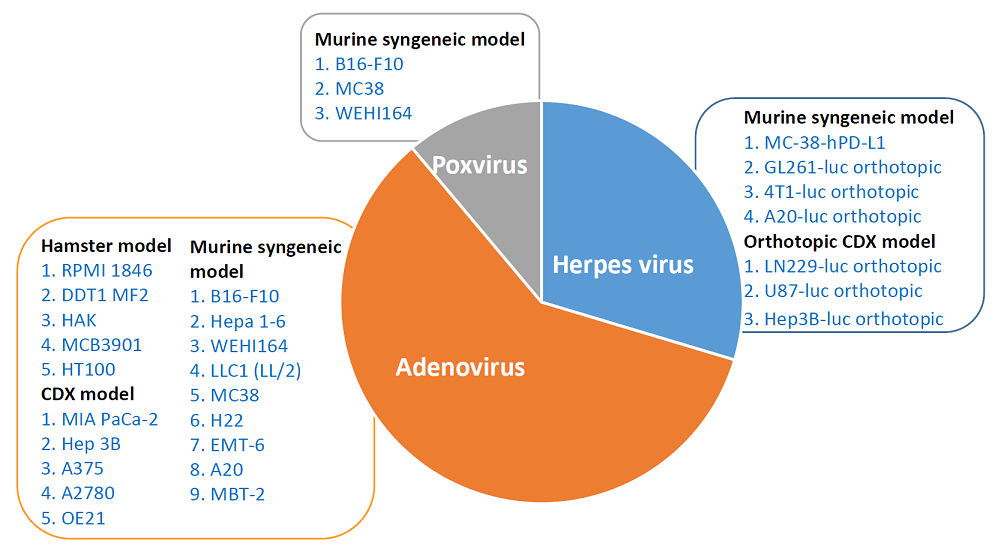
In vivo services
- Extensive portfolio of validated murine CDX and syngeneic models using both adenovirus-based and herpes virus-based OVs
- Study of efficacy and combination activity of OVs using CDX and syngeneic rodent models
- Analysis of biodistribution
- Assessment of viral shedding
- Immunogenicity and immunotoxicity evaluation
- Profiling of infiltrating immune cells
- Toxicology and safety studies
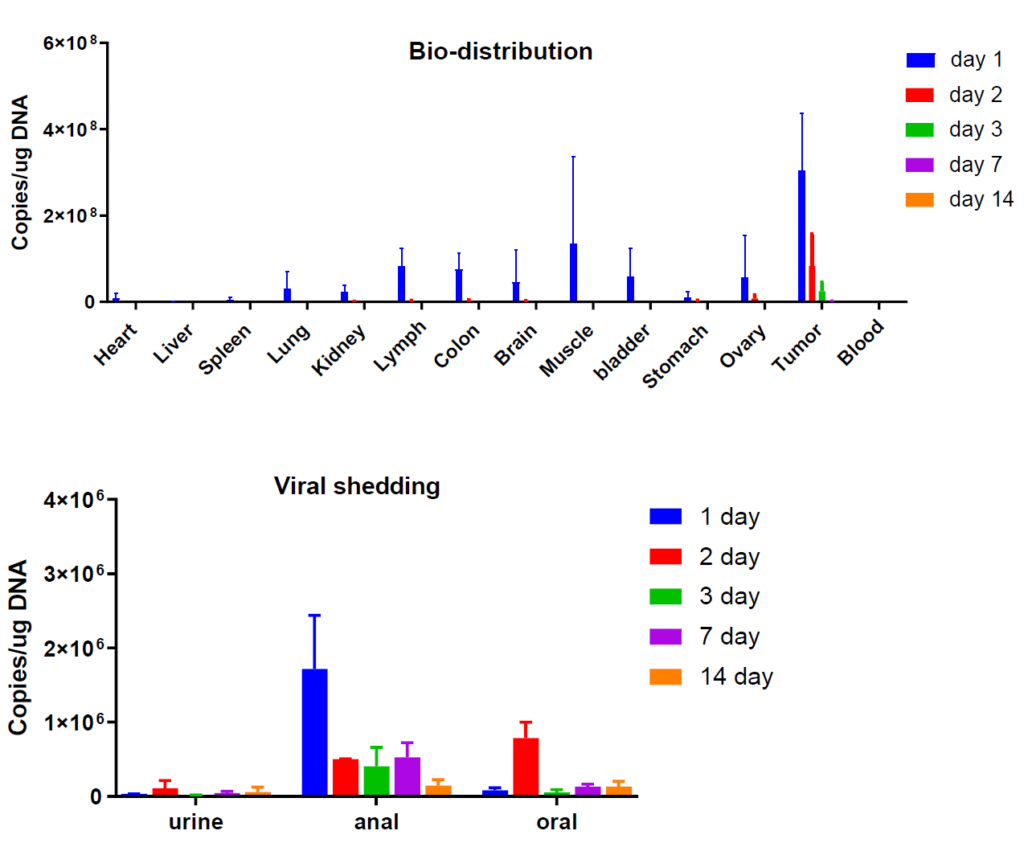
Bio-distribution and viral shedding assays

