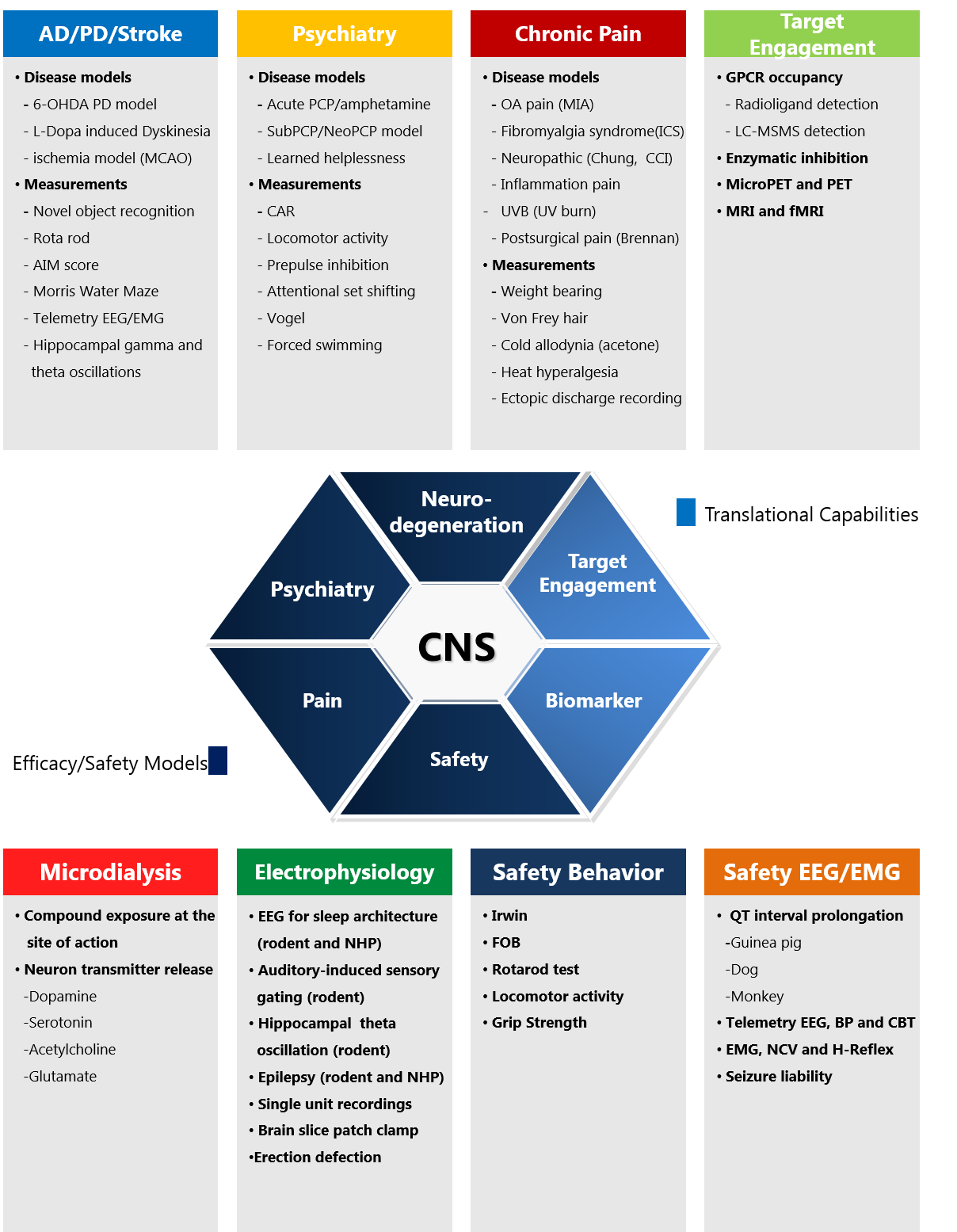
Neuroscience Drug Discovery
WuXi Biology offers an innovative technology & platform with integrated neuroscience capabilities with functional platform and leading edge science/technology to provide solutions that impact client’s portfolio.
- Target Discovery & Validation,
- Biomarker Discovery,
- Neuroscience focused Hit ID & Lead Discovery
- In vivo Pharmacology & Disease Models
- Ex vivo, IHC, ADME, PK/PD, Safety
The CNS & Pain Discovery team provides customer oriented integrated services within our AAALAC accredited animal facility and world class laboratories including a GLP standard laboratory. We strive for highest data quality, consistency, and maximal efficiency.
Best-in-Class Capabilities
- Functional leaders with disease and/or project leading experience
- Technical leaders with extensive bench experience
- Dedicated staff with a good training system
- International standard instrumentation and SOPs
- Multiple backups and new capacity expansion on-going
- Functionally diversified with validation of GPCR, Kinase and ion channel targets
New Modalities Capabilities
- AAV
- mRNA
- Oligonucleotide
In vitro CNS Platform Overview
- Assay development, screening and SAR support for GPCRs, transporters and ion channels
- Cell line development
- CNS-related GPCR binding and functional assays
- Microglia, astrocytes biology and functional assays
- Neuroprotective assessment in neurotoxcin induced cytotoxicity assay using neuronal cell line, rodent primary neuron, or rodent primary glia
- Abeta, glutamate, glucose deprivation, OGD, LPS etc.
- Readout as cell viability, apoptosis, inflammatory gene expression etc.
- Evaluation of neurite outgrowth in differentiated PC12 cell line or rodent primary neuron, neuronal iPSC
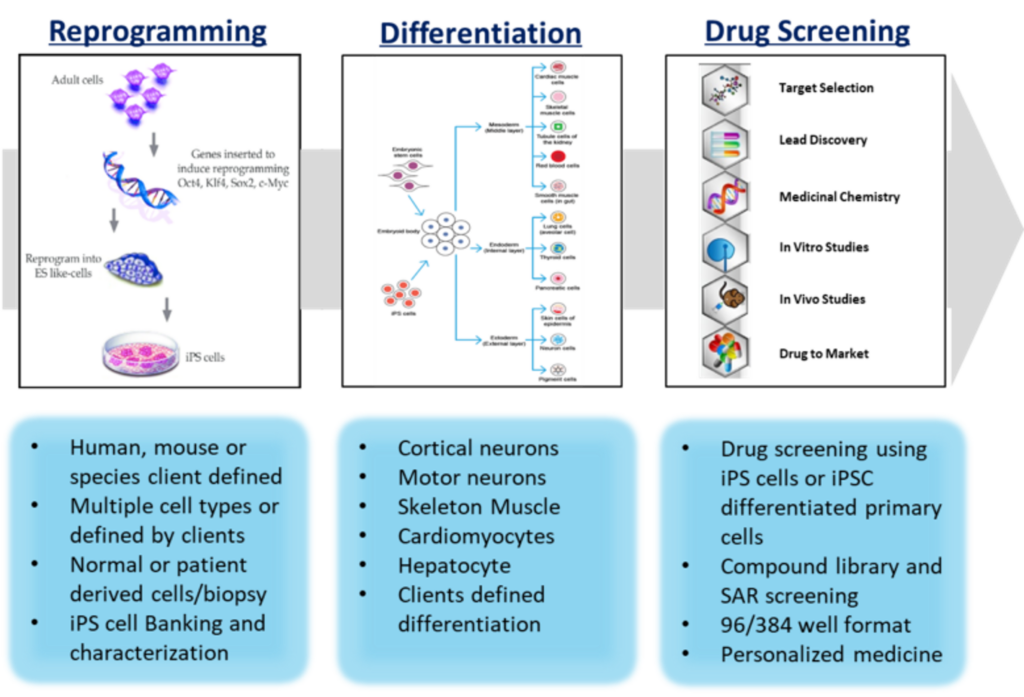
- Comprehensive iPSC platform: integrated capabilities and services including iPSC reprogramming, differentiation and drug screening.
- Target deconvolution using rodent brain tissue, neuronal cell line or rodent primary neuron etc.
Neuroscience target-based assays
Voltage and ligand Ion channels
- Over 50 stable cell lines
- Automated and manual patch-clamp assays
- Radioligand binding assays
Transporters
- Uptake assays
G-protein coupled receptors (GPCR)
- Over 120 stable cell lines
- Functional assay and binding assay
Enzymes
- Strong expertise of enzymology in drug discovery
iPSC Capability
Electrophysiology
Current capacity
- IonWorks Quattro: Using 384-well PatchPlates. Daily capacity of around about 3000 data points
- QPatch systems: Two QPatch machines, generating up to 60 concentration-response curves per day.
- Manual patch-clamp for recording from cultured cells: Four rigs in place, current capacity is about 10 concentration-responses curves per day
- Brain slice recording: One rig is available, capacity is 2-6 cells per day
Ion channel targets expansion
- Stable cell lines:
- HERG-HEK
- 1-CHO
- P2RX7-CHO
- TRPV1-HEK
- Nav1.4-CHO
- Nav1.6-CHO
- Nav1.7-CHO
- Final constructs:
- Nav1.8
- nAChR
- 7-Ric3
- Stim1/Orai1
- Stim1/Orai3
- Kir3.1-3.4
- TRPV4
in vivo CNS and Pain Studies
WuXi Biology offers strong capabilities with:
- Animal modeling from pharmacological and neurosurgical models to vector-based gene delivery, and of germline-transmitted genetic manipulations (Transgenic/knockout)
- Comprehensive behavioral characterizations from general behavior, motor function, learning and memory, pain behavior, to battery test-based disease-specific behaviors
- Full-scale molecular characterizations from gene/protein expression/profiling, epigenetic profiling and regulations, disease-specific biomarkers
- Receptor occupancy studies to measure CNS target engagement of free ligand binding to its intended receptor in vivo at the site of action
- Comprehensive histological studies from cell death, brain morphometry, disease-specific hallmarks, various specific staining, neuronal loss/gliosis, to synaptic morphology
- Experienced Team: leaders and key personnel are returnees with strong expertise in CNS areas and rich experience in both academia and industry
- Mature Infrastructure
- AAALAC-accredited animal facility,
- Histological/morphological/imaging facility
- Behavioral core facility with statistics support
- Multiple cell culture rooms for in vitro, ex vivo studies
- Bio-analytical labs (molecular biology, neurobiochemistry, LC-MS and others)
CNS Diseases Models
Neurodegenerative Diseases
- Alzheimer’s Disease (AD)
- Parkinson’s Disease (PD)
- Amyotrophic lateral sclerosis (ALS)
Psychiatric Models
- Anxiety
- Depression
- Schizophrenia
other disease models
- Multiple Sclerosis
- Epilepsy
- Stroke
- Aging Related Diseases
- Osteoporosis, etc.
Pain
- Acute inflammatory pain
- Post surgery pain
- Chronic inflammatory pain
- Acute nociception
- Chronic neuropathic pain
- Fibromyalgia-like pain
- Osteoarthritis, etc.
Behavioral Models
Experimental Psychiatric
- Anxiety-like behavior
- Depression-like behavior
- Schizophrenia-like behavior
- Bipolar-like behavior, etc.
Cognitive
- Learning and memory
- Spatial learning and memory
- Working memory
- Reference memory, etc.
Addiction
- Drug addictive behavior
- Drug-seeking behavior, etc.
Social Interaction
- Three-chamber sociability
- Social novelty test
- Tube dominance test, etc
Pain
- Pain-related behavior
- Spared nerve injury(SNI)
- Chemotherapy induced peripheral neurotoxicity(Cisplatin), etc.
Sensorimotor
- Motor function
- Neurological Testing, etc.
In Vivo Pharmacology Models & Translational Science
Due to the importance of translational science in CNS/pain drug discovery, we aim to set up the capability to help the success of clinical phase II. So far, we have developed a rat MIA model measured with weight-bearing which is considered to be the most relevant animal model for osteoarthritis. A receptor occupancy method was also developed to provide target engagement evidence.
Besides translational science, we have a built broad spectrum of animal models for CNS diseases and chronic pain to support a systematic approach in vivo drug discovery and development with multiple endpoints including: behavior, efficacy, PK/PD, safety, biomarker assays, and pathology.
- Monosodium iodoacetate (MIA)
- In vivo brain receptor occupancy measurement
Receptor Occupancy
- Assess CNS target engagement by measurement of free ligand binding to its intended receptor in vivo at the site of action.
- Supporting discovery program by providing confidence on compound MOA.
- Providing bases for the dose-regimen of preclinical and clinical studies
- Radio-labeled ligand detection by scintillation method
- Nonradioactive tracer detection by LC-MS/MS
- Full DRC of compound in rats or mice
Dose-dependent dopamine D2 receptor occupancy by Haloperidol in vivo:
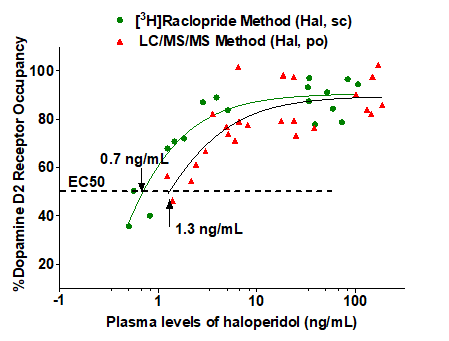
D2 receptor occupancy of haloperidol as determined by the two methodsData were presented as % occupancy vs. plasma exposure in individual animals. ED50 presented as mean of the group (n=3-5)
Pharmacological, Molecular and Biochemical Assays
- Distributional and quantitative analysis of gene expression at the mRNA and protein levels: real-time RT-PCR, microarray, in situ hybridization, Western blot, immunostaining, and ELISA.
- in vivo and in vitro isotope-labeled ligand binding/incorporation assay: receptor binding assay, autoradiography
- Brain regional drug delivery (stereotaxic microinjection) and chronic brain regional drug delivery (Osmotic mini-pump micro-infusion)
- Spatial and temporal epigenetic analysis: histone modification, DNA methylation, and DNA methylation profile.
- Microdialysis (under development)
Histological & Morphological studies
- Gross brain morphometry
- Colorimetric staining: Nissl staining (neuron), LFB staining (glial myelin), Golgi staining (dendritic spine), Schiff staining, HE staining etc.
- Immunostaining, confocal microscope-based double/triple immunostaining (collaboration with local institutes)
- Adult neurogenesis;
- Dendritic spine/synapse morphology
- Subcellular fraction
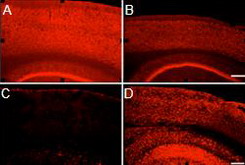
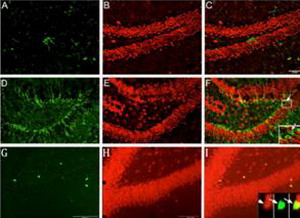
A-F. Double immunostaining of NeuN (red) and
DCX (blue) shows cell proliferation. G-I. Double immunostaining of NeuN (red) and BrdU (green)
shows adult neurogenesis in the dentate gyrus.
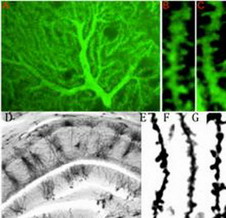
Neurabin-based immunostaining of cerebellar Purkinje’s cell in the mouse. D-G. Golgi impregnation staining of the hippocampus in
the mouse.

