CAR-T Cell Therapies Platform
Chimeric antigen receptor (CAR)-T cell therapy represents a major advancement in personalized cancer treatment. This therapy has shown remarkable results with hematological malignancies (including anti-CD19 CAR-T therapy against B cell malignancies). To help accelerate research in this field, we provide a comprehensive platform of in vitro services and in vivo animal models for supporting project teams focused on the discovery of CAR-T cell therapies.
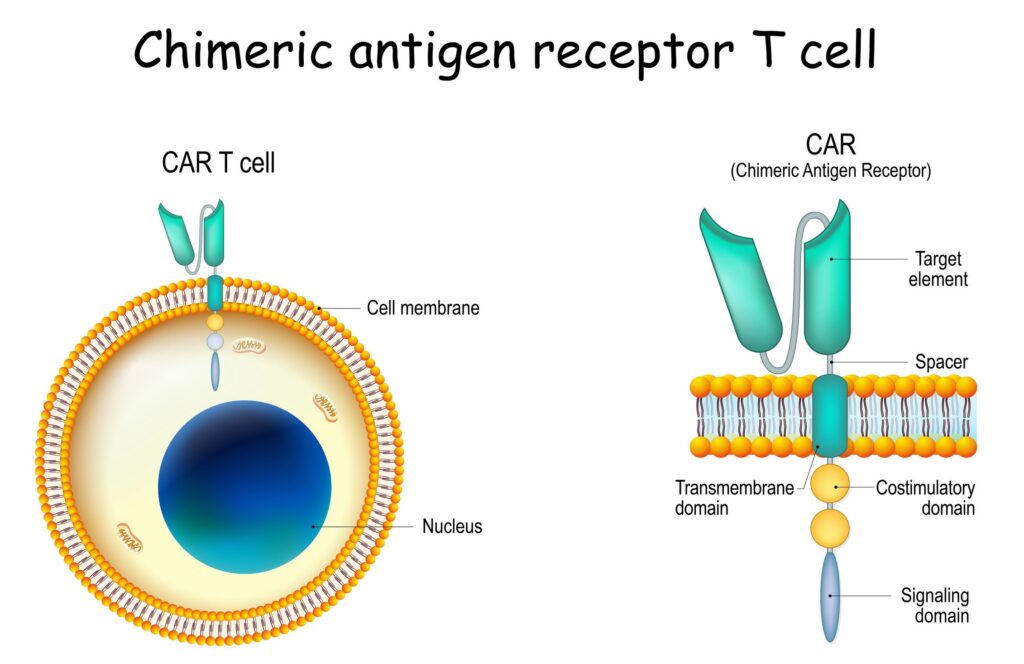
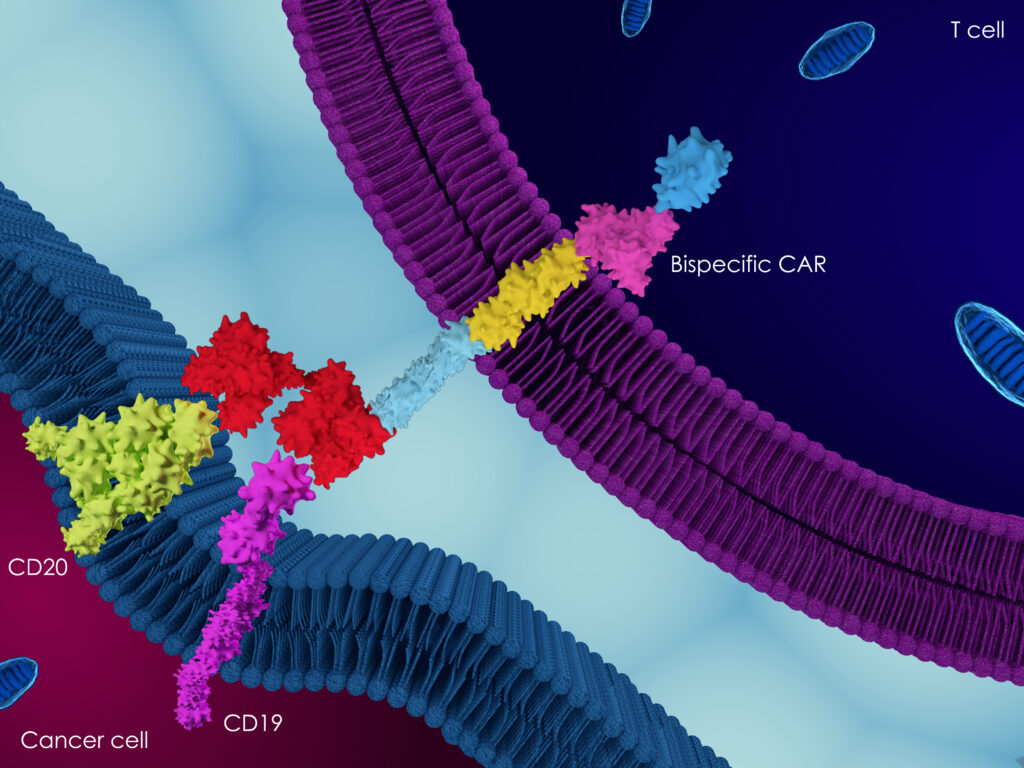
In vitro Assays and Services
- CAR design and construction (sequence optimization, lentiviral vector selection)
- Packaging of viral particles (titration, viral copy number)
- Optimization of transfection efficiency of CAR-T cells with lentiviral transduction enhancers
- Functional evaluation, including cytotoxicity testing (co-culturing CAR-T cells and tumor cells) and measurements of cytokine release
- Optimization of culture media and conditions for CAR-T cell proliferation
- Analysis of CAR expression via flow cytometry
- Expansion of CAR-T cells and phenotype characterization (differentiation of T cells)
- Biomarker services (multiplexed tissue staining)
- Safety QC assessment (endotoxin, mycoplasma)
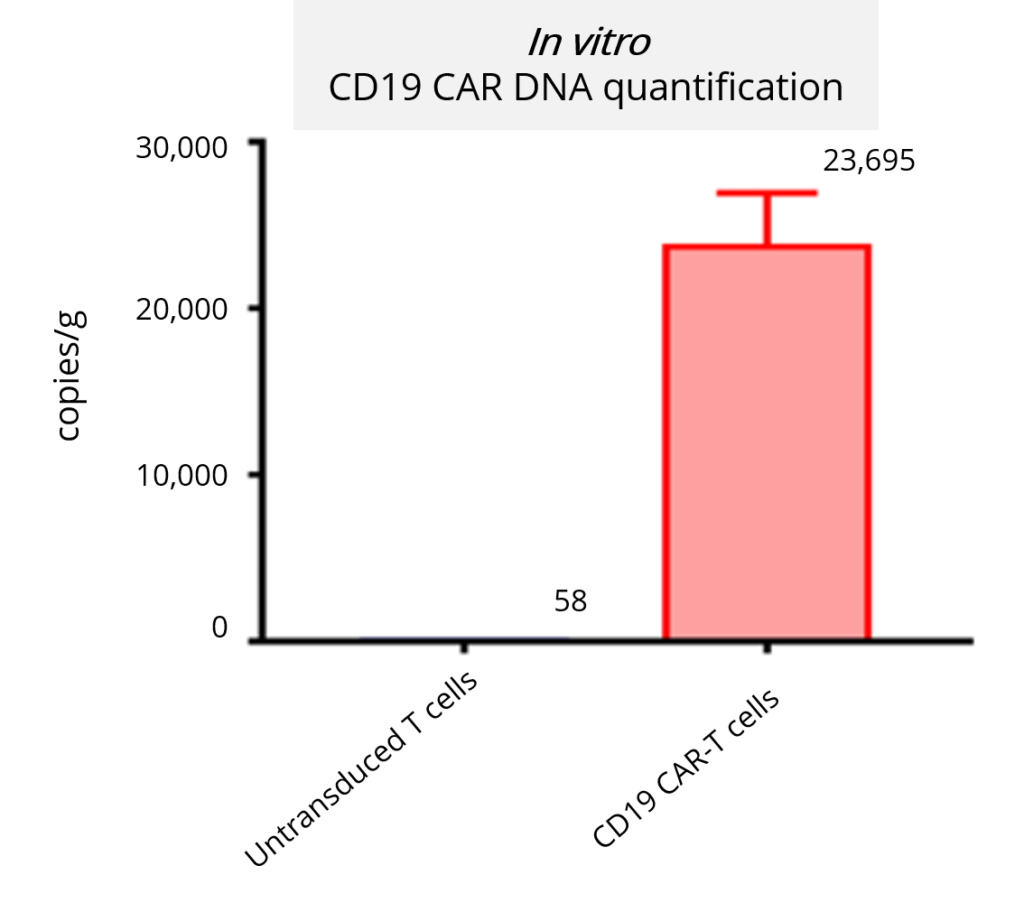
In vivo Animal Models
- Extensive portfolio of PDX models, with biomarkers
- Panel of more than 280 CDX models, covering 29 tumor types
- Syngeneic models
- Orthotopic and metastatic models
- PBMC or HSC humanized models
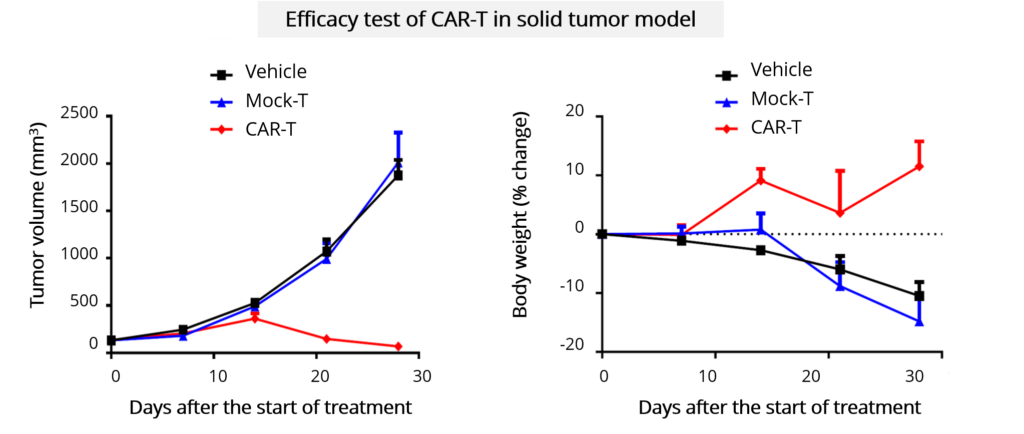
In vivo Efficacy
| In vivo anti-tumor efficacy | • Endpoints: Tumor volume measurement for subcutaneous models; bioluminescence imaging for systemic models and orthotopic models; survival analysis • Clinical Observation: Food consumption, body weight, behavior and morphology, necropsy at the endpoint |
| CAR-T viability and biodistribution | • In vivo proliferation, survival, and long-term persistence of CAR-T cells • Tumor or tissue distribution and infiltration of CAR T cells • T cell phenotype analysis (Memory and effector T cells, Tregs) |
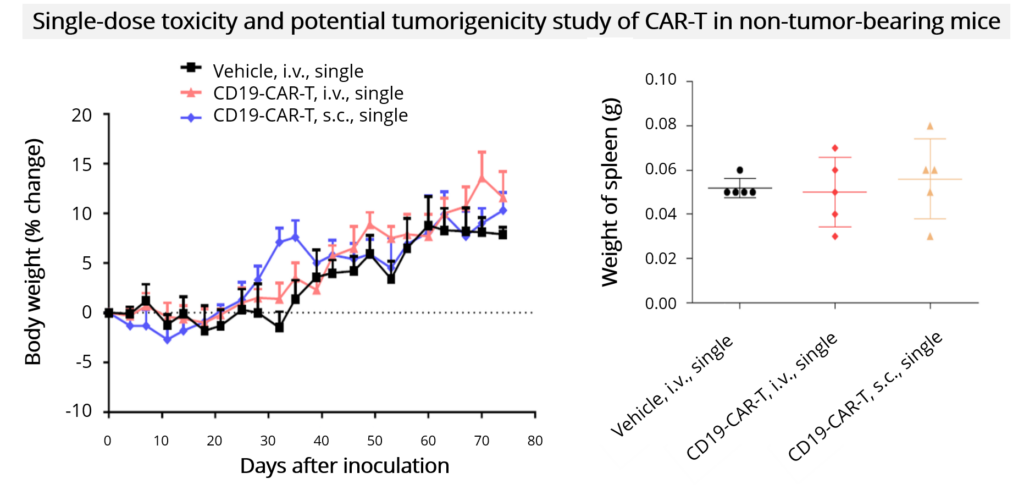
| Toxicity evaluation (non-GLP) | • Cytokine release analysis • Single dose toxicity • Potential tumorigenicity of CAR-T cells |
- Tumor volume measurements (for subcutaneous models)
- Bioluminescence imaging (for systemic and orthotopic models)
- Survival analysis
- Clinical observations (including necropsy at endpoint)
- Infiltration of CAR-T cells in tumor and tissue
- Proliferation, survival, and long-term persistence of CAR-T cells
- T cell phenotype analysis (e.g., memory and effector T cells, Tregs)
- Toxicity assessment (including single-dose toxicity and cytokine release)
Related Resources View All
Armoring CAR-T Therapy with PD-1 Blockade
Resource Type: Latest Science Poster
AACR 2024 Posters: Sneak Peek
Resource Type: Article Blog
Pioneering Advanced Modalities in Early Drug Discovery
Resource Type: Article
Control of the Antitumor Activity and Specificity of CAR T...
Resource Type: Latest Science Publication
PD-1 Blockade Protein in 4th Generation Armored CAR-T Cells Enhances...
Resource Type: Latest Science Poster
WuXi AppTec Discovery Services
Resource Type: Brochure

