Protease Assay
Protease Drug Discovery Services
The human genome contains over 600 protease genes that contribute to fulfill the “degradome” functionality [1]. Moreover, it has been realized that the functionality of proteases goes far beyond non-specific degradation of multiple substrates, as in the case of proteinase K, since many proteases, such as the secretases that process the amyloid beta precursor protein APP, have been shown to cut at precise sites and catalyze specific proteolytic reactions.
Dysregulation of the proteolytic system is associated with many pathologies, such as cancers, neurodegenerative diseases, and cardiovascular diseases. Small-molecule drugs that target specific proteases, such as angiotensin converting enzyme (ACE), HIV protease, and thrombin, have been developed for the treatment of hypertension, AIDS and thrombosis, respectively [3].
To support the discovery of protease drugs, WuXi Biology offers a comprehensive platform of services. With our strong expertise and cutting-edge technologies, we can provide services to cover a broad spectrum of proteases, including but not limited to targets such as aspartic proteases, serine proteases, cysteine proteases, metalloproteases and COVID-19-related protease assays (see table below). These services are provided with the options of IC50 determination or single-point test, and Kinact/KI determination. In addition, we also offer protease screening and profiling services, from a safety and efficacy perspective, to characterize candidate protease drugs.
| Class | Human protease |
| Aspartic protease | Cathepsin D, Cathepsin E, Renin, ACE1, ACE2, etc |
| Cysteine protease | Calpain-1, Caspase-1, Caspase-2, Caspase-3, Caspase-7, Caspase-8, Cathepsin B, Cathepsin K, Cathepsin L, Cathepsin S, Cathepsin G, etc |
| Metallo protease | MMP1, MMP2, MMP3, MMP7, MMP8, MMP9, MMP10, MMP13, MMP14, MMP15, MMP16, MMP17, Neprilysin/CD10, rhACE-2, etc |
| Serine protease | Cathepsin A, Chymase, Chymotrypsin C, DPP4/CD26, Elastase, Activated Protrein C (aPC), Factor IXa, Factor VIIa, Factor XIa, Factor XIIa, Neutrophil Elastase, Factor Xa, plasmin, Plasma Kallikrein, Tissue killikrein-1, Thrombin, Trypsin-1, Tryptase β, DPP8, DPP9, Tissue Plasminogen Activator (tPA), Urokinase (uPA), etc |
| Other protease | DDP3, DDP4, DDP7, DDP8, DDP9, ubiquitin-specific proteases (such as USP7, USP14, etc) |
Case Studies
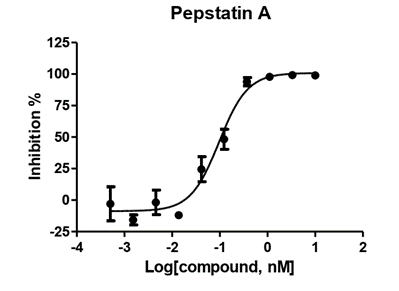
Aspartic protease
- rhCathepsin E: 0.2nM
- Substrate: 10µM
- 30 minutes pre-incubation
- Enzymatic reaction time: 40 minutes
- Format: 384 well
- Assay window: >7 folds
- Z factor:>0.6
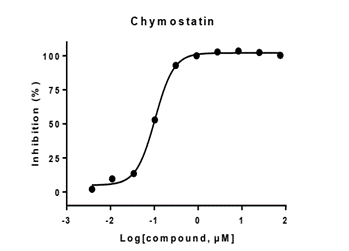
Serine protease
- Enzyme: recombinant human CTRC
- Reference: chymastatin
- 30 minutes pre-incubation
- Enzymatic reaction time: 60 minutes
- Format: 384 well
- Assay window: >3 folds
- Z factor:>0.7
Cysteine protease
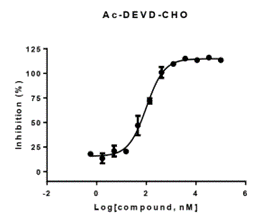
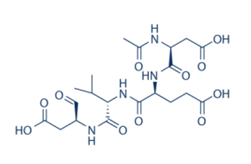
- Enzyme: recombinant human caspase 1
- Reference: Ac-DEVD-CHO
- 30 minutes pre-incubation
- Enzymatic reaction time: 60 minutes
- Format: 384 well
- Assay window: >7 folds
- Z factor:>0.7
Metalloproteases
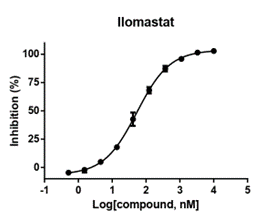
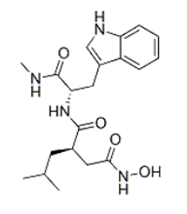
- Enzyme: recombinant human MMP3
- Reference: Ilomastat
- 30 minutes pre-incubation
- Enzymatic reaction time: 60 minutes
- Format: 384 well
- Assay window: >4 folds
- Z factor:>0.6
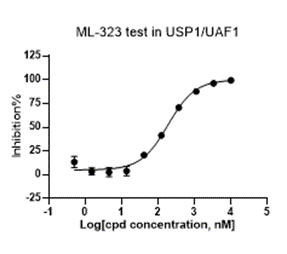
Deubiquitinases
- Enzyme: USP1
- Reference: ML-323
- 10 minutes pre-incubation
- Enzymatic reaction time: 30 minutes
- Format: 384 well
- Assay window: >10 folds
- Z factor:>0.5
References:
[1] Bond JS. Proteases: History, discovery, and roles in health and disease. J Biol Chem. 2019 Feb 1;294(5):1643-1651. doi: 10.1074/jbc.TM118.004156.
[2] López-Otín C, Bond JS. Proteases: multifunctional enzymes in life and disease. J Biol Chem. 2008 Nov 7;283(45):30433-7. doi: 10.1074/jbc.R800035200.
[3] Turk B. Targeting proteases: successes, failures and future prospects. Nat Rev Drug Discov. 2006 Sep;5(9):785-99. doi: 10.1038/nrd2092.
[4] López-Otín C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007 Oct;7(10):800-8. doi: 10.1038/nrc2228.

