Quantum Mechanics Quiz part II
QM Magic Class | Chapter 47
< Magical Power of Quantum Mechanics
Zhifa Pu, Qiuyue Wang, Hailong Ren, Wenfeng Liu, Tommy Lai, Yongsheng Chen, John S. Wai
This chapter covers four questions we had in an open-book QM examination in WuXi AppTec. For setting up QM calculations and interpreting results, participants have access to our QM e-Book [1] and Spartan manual [2]. They were requested to refrain from doing literature search for answers during the 2-hour exam. The analyses below are provided by our selected participants.
Question 1 | The Chan-Lam reaction below formed a single product, II or III? What are the QM parameters to calculate for analysis? Please provide and interpret QM calculation results.
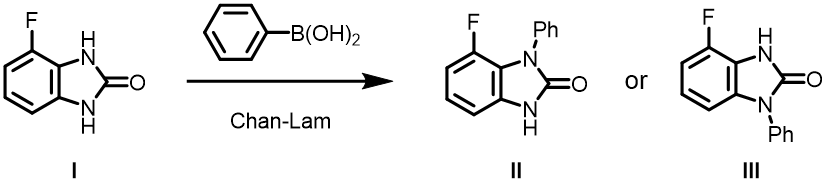
Answer (Zhifa Pu)
For the Chan-Lam reaction[3], first we need to find out which NH is more acidic. Based on the calculated Electrostatic Potential Map (ESP Map) of substrate I, N1-H is more acidic (N1-H: ESP 250.6 kJ/mol > N3-H: 236.1 kJ/mol) and will be selectively deprotonated. Intuitively, one would have expected N3-H, next to the fluoro group, to be more acidic.
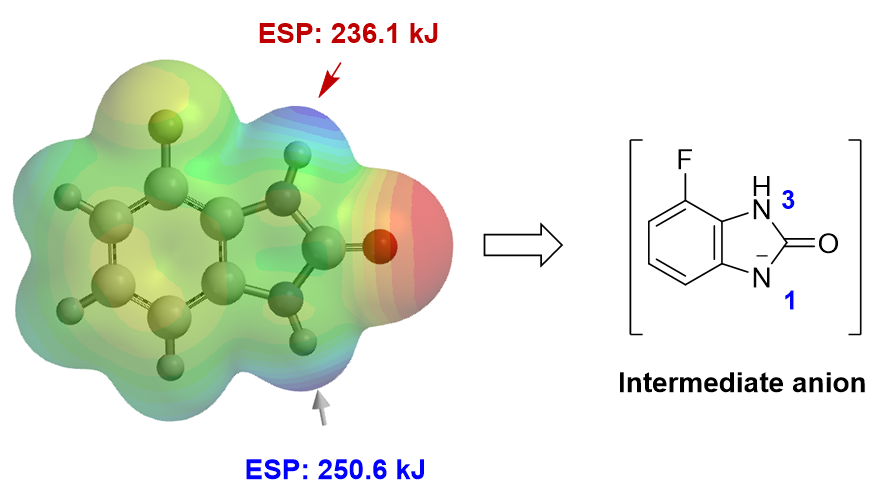
Next, we calculate for HOMO of the resultant anion, which shows the necessary lobe for N1 phenylation. As such, Chan-Lam reaction of compound I should occur preferentially at N1 to generate a single product III.
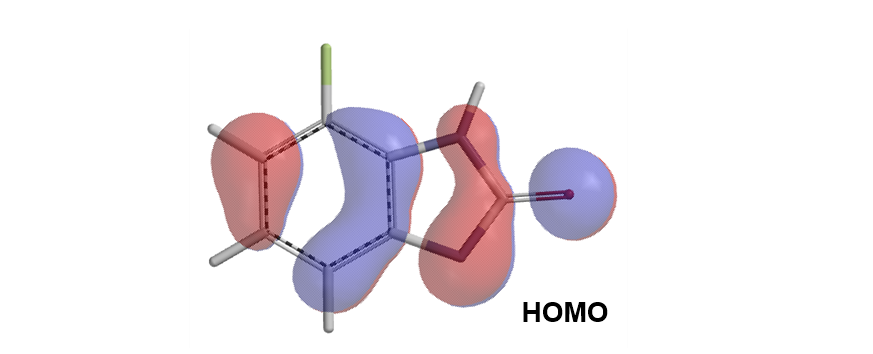
Question 2 | The below Sonogashira reaction formed a single product, it is II, III or IV[4]? What are the QM parameters to calculate for analysis? Please provide and interpret QM calculation results.
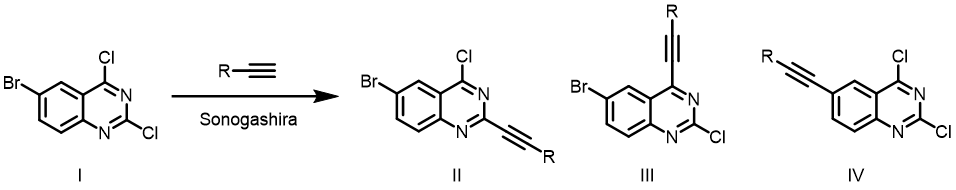
Answer (Qiuyue Wang)
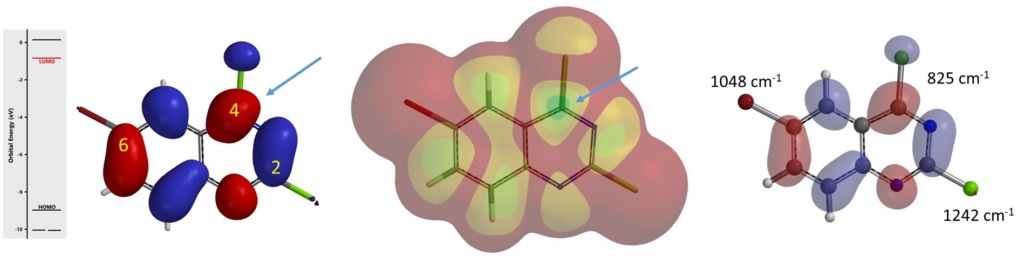
For Sonogashira reaction, like other metal-catalyzed cross coupling reactions, regioselectivity is controlled by the oxidative addition step. Shown in figure 3 are the LUMO and LUMO Map of substrate I, where only C4 has an obvious LUMO lobe centered on the C-halogen carbon. LUMO and LUMO+1 energy gap is relatively large (0.98 eV), so we do not need to consider LUMO+1. We also use QM calculated C-X bond infrared stretching vibrational wavenumbers to differentiate their relative reactivity toward oxidative addition. The weaker the C-X bond is, the lower the wave number it has, and the easier it is for the bond to undergo oxidative addition. From the calculation, C2-Cl, C4-Cl and C6-Br bond stretching wavenumbers are 1242, 825, and 1048 cm-1, respectively, with the C4-Cl one being the weakest bond. Both methods predict that oxidative addition will proceed selectively at C4 and lead to a single product III [5].
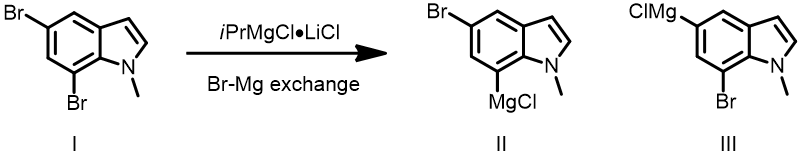
Question 3| The below bromide-magnesium exchange reaction formed a single product, II or III[6]? What are the QM parameters to calculate for analysis? Please provide and interpret QM calculation results.
Answer (Hailong Ren)
For polyhalogenated substrates, we look at LUMO and LUMO Map, compare the calculated stretching wavenumber of the carbon-halogen bonds to correlate with regioselectivity of SnAr and oxidative addition reactions. With halide-lithium exchange or halide-magnesium exchange reactions we discovered that it is more appropriate to compare appropriate LUMO+n with unique “String-of-Pearls” shaped lobes along the carbon-halogen bonds of the substrates [7].
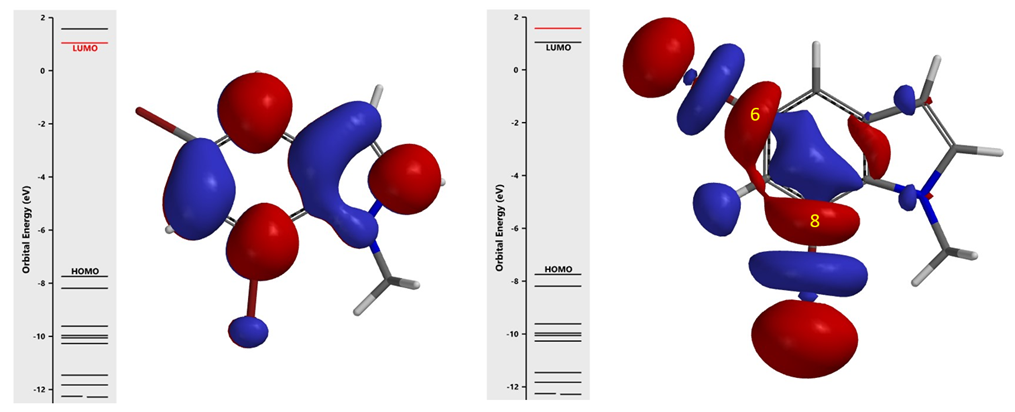
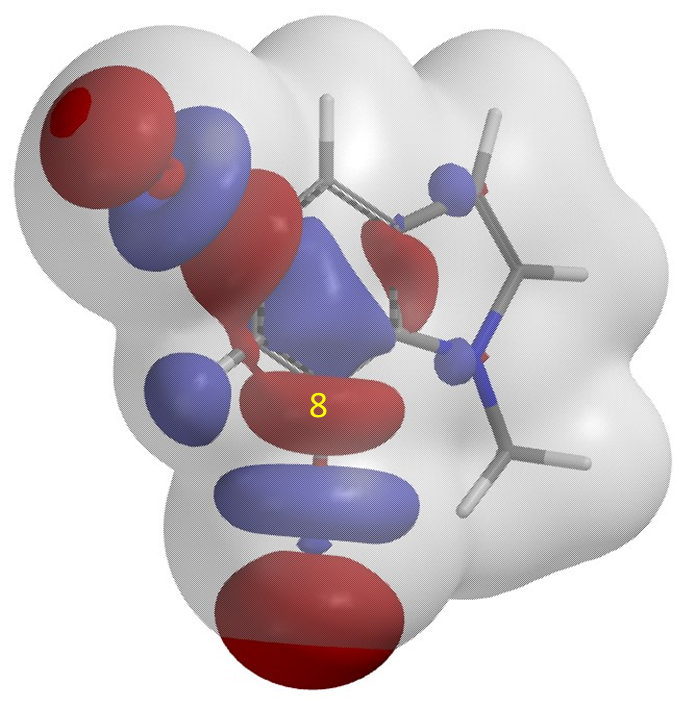
As shown in Figure 4, LUMO+1 of substrate I has the unique “String-of-Pearls” lobes distribution along the two C-Br bonds. Overlaying it with Electron Density Map (Figure 5) reveals that the C7-Br Lobe is more accessible for Grignard exchange; as such, the C7 bromo group will react preferentially to provide selectively product II. This example exemplifies a unique characteristic of halogen-metal exchange reaction, that is, due to the unique unoccupied orbitals required, with its terminal lobe extending beyond Electron Density isosurfaces, it is not sensitive to neighboring group’s steric hindrance[7].
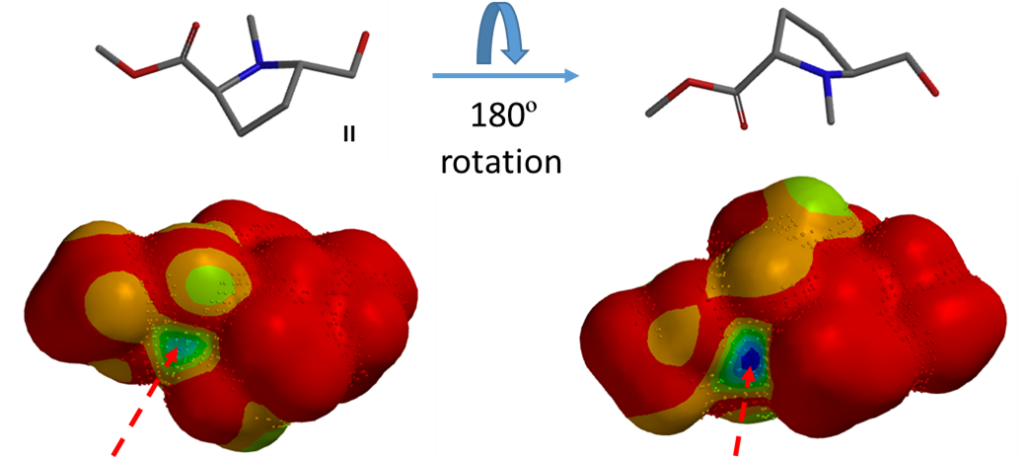
Question 4 | Reduction of diester I with sodium borohydride provides exclusively II as the only product[8]. What are the QM parameters to calculate for analysis to account for the selectivity? Please provide QM calculation results and interpret.
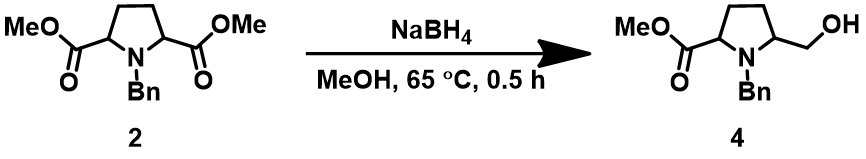
Answer (Wenfeng Liu)
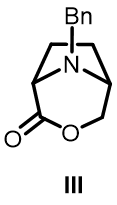
When one ester group is reduced first, only a single product II is obtained. Lactone III as shown in the figure above is not observed, indicating it is likely that the ester groups are trans to one another.
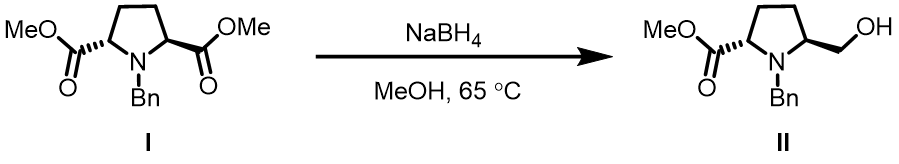
Based on this assumption we calculated for the LUMO map of substrate I and added to it inaccessibility markers. The blue to green coloration on LUMO map at the carbonyl carbons, on both sides of the molecule, represents decent values of the orbital, yet only one of them and only from one side is accessible for reduction to provide compound II.
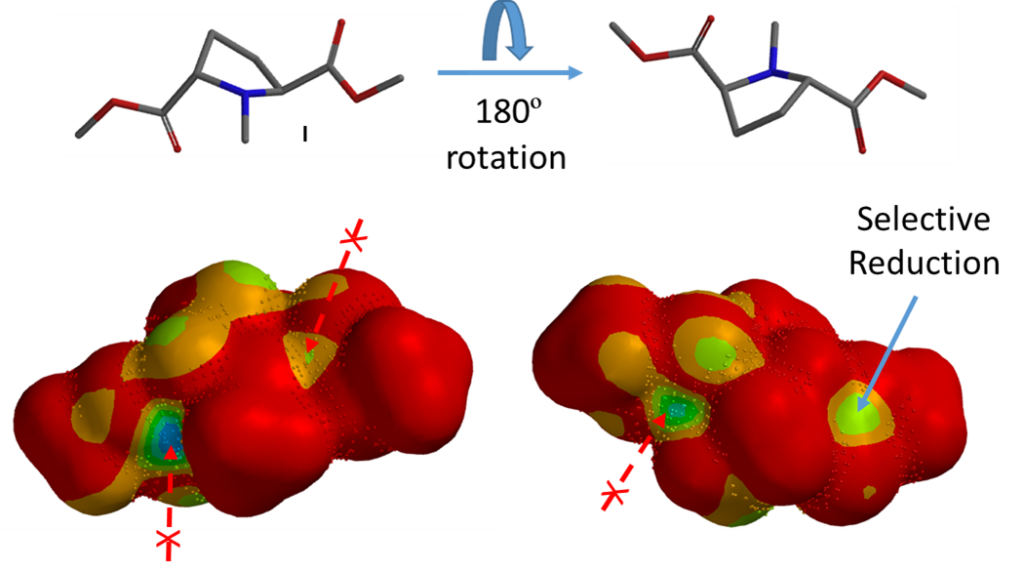
LUMO Map calculated for structure II reveals that the remaining carbonyl carbon is covered with inaccessibility markers, indicating that this carbonyl is not readily accessible for reaction, accounting for why product II cannot be further reduced under mild reduction conditions.
References:
[1] Quantum Mechanics for Organic Chemists: An Experimentalist Approach. https://wuxibiology.com/resource/quantum-mechanics-for-organic-chemists/
[2] Spartan Tutorial and User’s Guide. Accessible within Spartan software and from https://www.wavefun.com/spartan-documentation
[3] QM chapter 34 “QM Analyses of Regioselectivity in Chan-Lam Reaction” https://wuxibiology.com/qm-analyses-of-regioselectivity-in-chan-lam-reaction/
[4] I. Mangalagiu, T. Benneche, K. Undheim, Acta Chem. Scand. 1996, 50, 914
[5] QM chapter 45 “Unusual Reactivities of Polyhalogenated Heteroaromatic Substrates are Predictable” https://wuxibiology.com/unusual-reactivities-of-polyhalogenated-heteroaromatic-substrates-are-predictable/
[6] P. Anbarasan, H. Neumann, M. Beller, Chem. Eur. J. 2011, 17, 4217
[7] QM chapter 39 “Unraveling Divergence in Haloselectivity of SNAr and Cross Coupling vs Halogen-Metal Exchange Reactions” https://wuxibiology.com/unraveling-divergence-in-haloselectivity-of-snar-and-cross-coupling-vs-halogen-metal-exchange-reactions/
[8] K. W. Yang, L. Feng, S. K. Yang, M. Aitha, A. E. LaCuran, P. Oelschlaeger, M. W. Crowder, Bioorg. Med. Chem. Lett., 2013, 23, 5855

