QM Analyses of Regioselectivity in Chan-Lam Reaction
QM Magic Class | Chapter 34
< Magical Power of Quantum Mechanics
A Tianjin colleague raised a very practical question, how to use orbital analysis to predict regioselectivity of Chan-Lam reaction between indazole 1 and phenylboronic acid 2 (Figure 1).
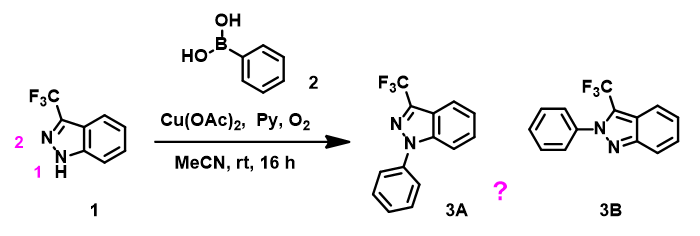
Figure 1. Chan-Lam reaction between indazole 1 and phenylboronic acid 2.
Mechanism of Chan-Lam Reaction
First question to ourselves became “shall we calculate for HOMO of the neutral form of the molecule or its anion for analysis?”. Shown on Figure 2 is the proposed mechanism for Chan-Lam reaction[1]. Arylboronic acid undergoes transmetalation with divalent copper complex E to form intermediate A, which coordinates with deprotonated heterocycles to form Cu(II) complex B, which undergoes air oxidation to form Cu(III) Intermediate C. Intermediate C undergoes reductive elimination to form coupling product and Cu(I) complex D, which is further oxidized to regenerate Cu(II) complex E. As such, deprotonation of the heterocycles and addition of the corresponding anion to form Cu(II) B are the crucial steps in the catalytic cycle. To understand selectivity of the reaction, we should look at HOMO of the anion, not HOMO of the neutral molecule.
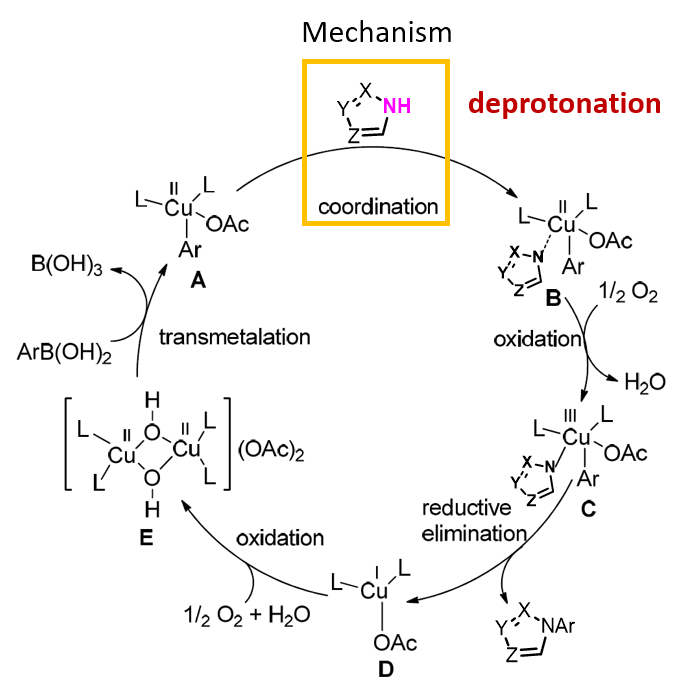
Figure 2. Possible mechanism of Chan-Lam reaction[1]
HOMO Analysis
Shown on Figure 3 is HOMO of indazole 1 anion. There is significant HOMO lobe on N1, but almost nothing on N2, suggesting that Chan-Lam reaction will occur preferentially at N1. The determining factor in this reaction is electronic effect, rather than the potential steric interaction from the CF3 group.
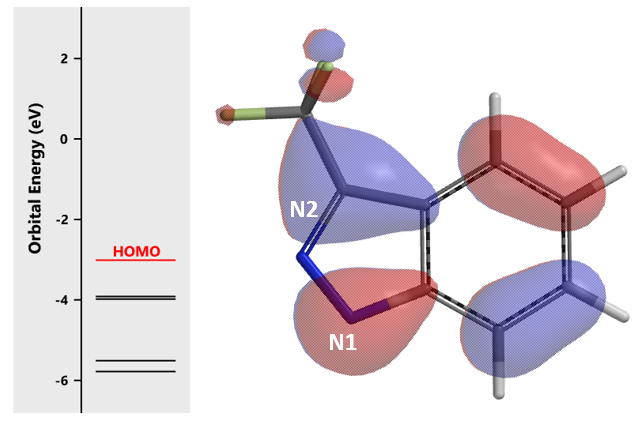
Figure 3. HOMO of indazole 1 anion
Experimentally, Tianjin colleague observed formation of N1 phenylated product 3A only, (Figure 4), lending support for the use of HOMO anion for prospective regioselectivity analysis of the reaction.
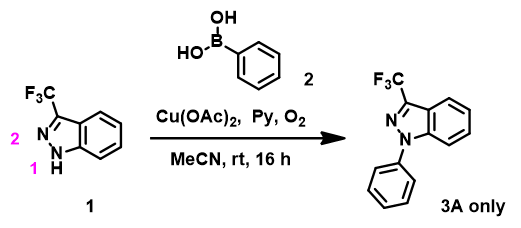
Figure 4. Chan-Lam reaction between indazole 1 and phenylboronic acid 2.
Further Testing of the HOMO anion Analysis
Next we collected additional examples from the literature for analysis[2]. Pyrazole (Table 1, entry 1), imidazole (entry 2), and benzimidazole (entry 7) do not require analyses for regioselectivity[2a]. HOMO of 1,2,3-triazole anion (Table 1 & Figure 5, entry 3) suggests Chan-Lam reaction will proceed predominately on N2, to a lesser extent on N1, in contrast to reported N1 arylation product only [2a]. Perplexing!
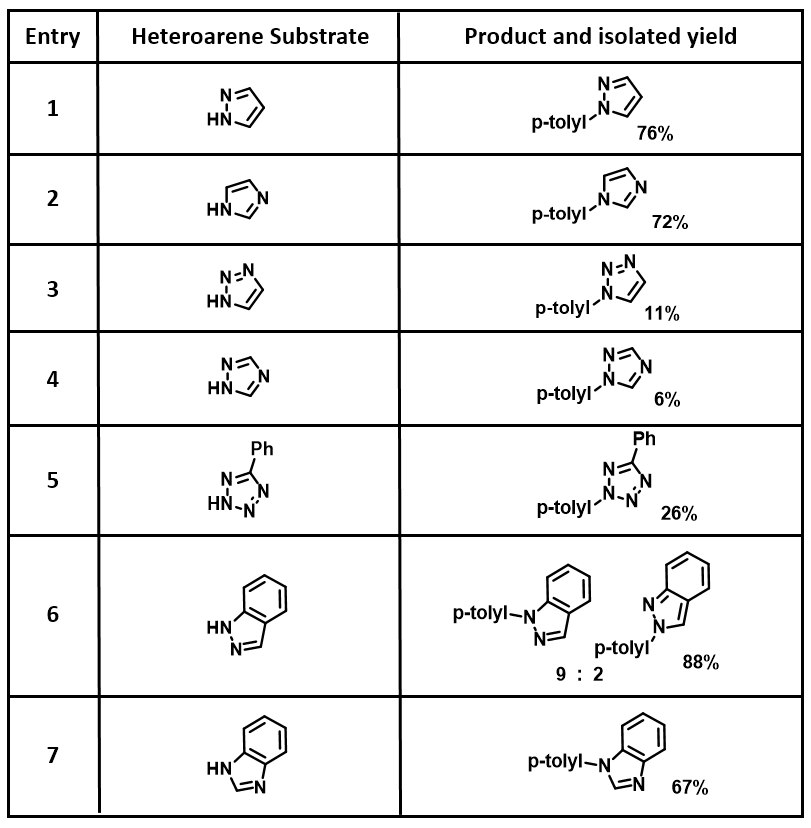
Table 1. Chan-Lam reaction between various heterocycles and p-tolylboronic acid[2a].
Moving down the Table, HOMO of 1,2,4-triazole anion (entry 4) suggests reaction to be on N1 or N2, consistent with the experimental result. For phenyl tetrazole (entry 5), HOMO lobes at four nitrogens are similar in size, while C5 phenyl group will hinder coordination with Cu(II) at N1 and N4 position. We expected reaction to occur mainly at N2 or N3 position, aligned with reported result. For indazole (entry 6) selective N1 phenylation was expected and observed. Regioselectivity for all other examples in the literature[2] are accounted for with HOMO anion analysis; the only exception is 1,2,3-triazole (entry 3) discussed above. We were curious and decided to repeat the experiment.
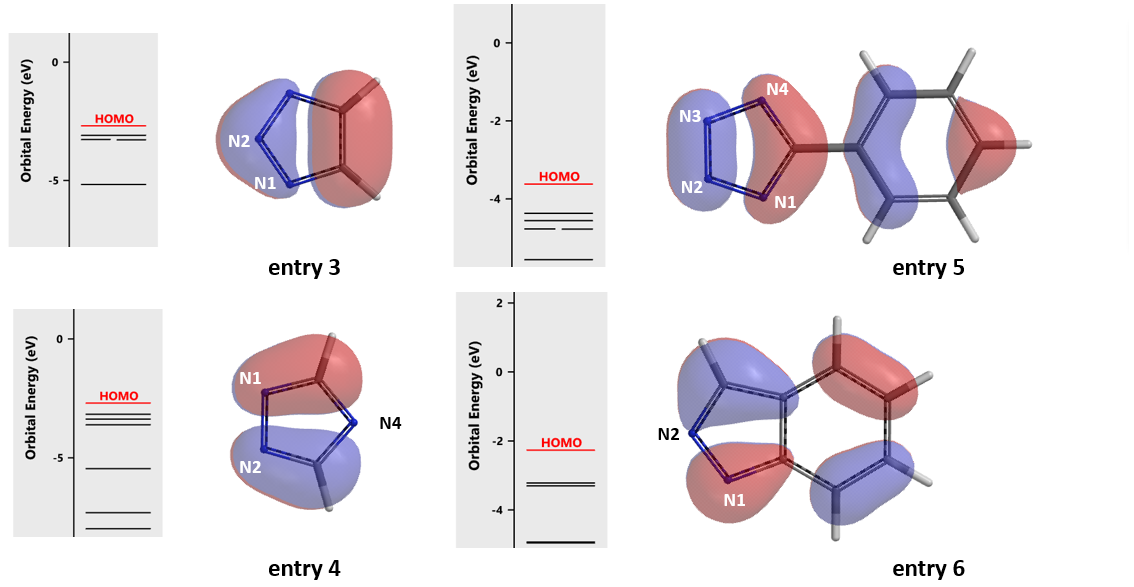
Figure 5. HOMO anion of entries 3 – 6.
Before running the reaction, we also calculated dipole moments for the two isomeric products and the two potential by-products (aryl ether and aryl acetate) as a measure of their polarities. The dipole moments of the N2 and N1 arylation products are 0.49 and 4.88 Debye, respectively (Figure 6). The N2 product is significantly less polar than N1 one, and is even lower in polarity than aryl ether and aryl acetate.
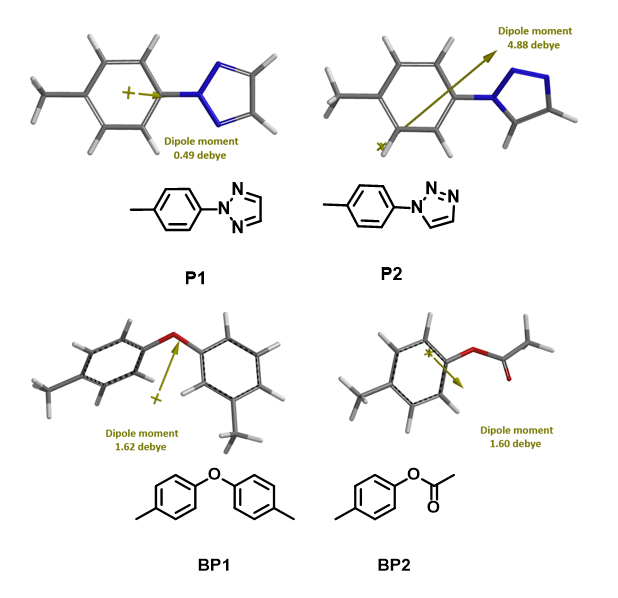
Figure 6. Dipole moment calculated for P1, P2, BP1, BP2.
Experimental Validation
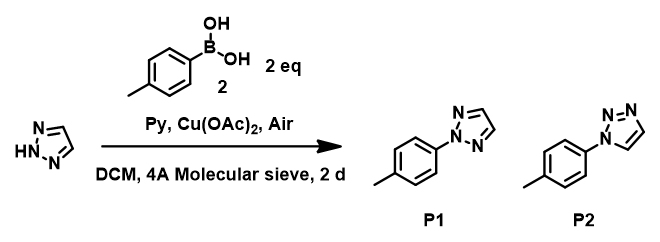
Figure 7. Chan-Lam reaction of 1,2,3-triazole and p-tolylboronic acid.
Reaction between 1,2,3-triazole and p-tolylboronic acid was repeated and products carefully separated and purified by column chromatography. We observed the formation of two products P1, P2 (~2 : 1), and two by-products BP1, BP2. 13C NMR of P1, major product, has six sets of signals, corresponding to N2 arylation, while P2, minor product, exhibits seven sets of signals, indicating arylation is at N1 position. These are further confirmed by comparing 13C and 1H NMR shifts of both isomers reported in the literature [3],[4], further validating the prediction with HOMO analysis for 1,2,3-triazole anion, resolving the puzzle (Figure 8).
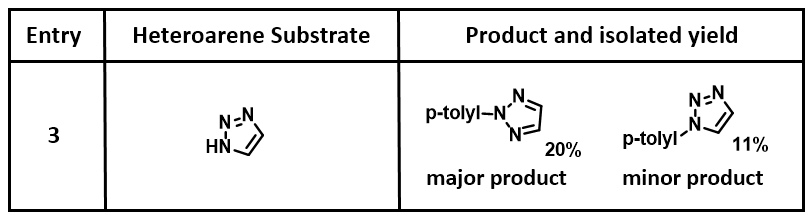
Figure 8. Major and minor products from Chan-Lam reaction between 1,2,3-triazole and p-tolylboronic acid
During purification, we found that the polarity of P1 is indeed much lower than that of P2, and lower than that of the two by-products BP1 and BP2, consistent with the calculated dipole moments. These parameters provided useful guidance in locating and purifying all four reaction products (Figure 6).
Conclusion
For Chan-Lam reaction, HOMO of substrate anion is a useful model for prospective analysis of regioselectivity. Chan-Lam reaction of 1,2,3-triazoles provides ~2 : 1 N2 vs N1 arylation products, consistent with the QM analysis.
Building on What We Just learned
Chan-Lam Reaction is sensitive to the nature of heterocyclic substrates[2a]. For example, pyrrole and indole failed to react with p-tolylboronic acid, and the more acidic triazole and tetrazole did not react well. We learned that deprotonation of the NH proton on the heterocyclic ring and the coordination of the resultant anion onto Cu(II) A are crucial steps in the reaction. Tabulated on Table 2 are pKa of heterocyclic substrates and isolated yields for the reaction under standard conditions (pyridine as base and ligand at 25ºC). From your experience, what pKa range the heterocyclic substrates need to fall within for Chan-Lam reaction to be feasible?
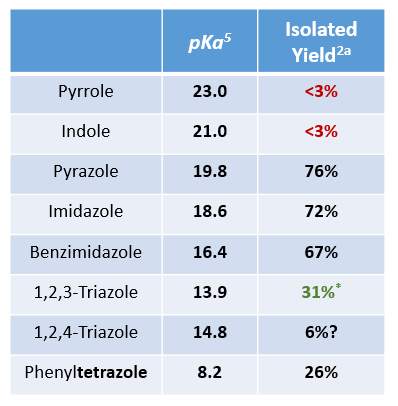
Table 2. Isolation Yield [2a] and pKa of Heterocyclic Substrates [5] * 31% (overall yield of P1 and P2)
This article is written and edited by Xiaocang Wei, Qiuyue Wang, Jian Wang, Yongsheng Chen, John S. Wai
References:
[1] X.P. Ma, F.P. Liu, D. L. Mo, Chinese J. Org. Chem., 2017, 37, 1069.
[2] (a) P.Y.S. Lam, C.G. Clark, S. Saubernt, J. Adamst, M.P. Winters, D.M.T. Chan, A. Combst Tetrahedron Lett. 1998, 39, 2941; (b) D.M.T. Chan, P.Y.S. Lam, in Boronic Acids, Ed.: Hall, D. G., Wiley-VCH, Weinheim, 2005, Chapter 5, p205 and references therein cited; (c) J.X. Qiao, P.Y.S. Lam, in Boronic Acids, Ed.: Hall, D. G., Wiley-VCH, Weinheim, 2011, Chapter 6, p315 and references cited therein.
[3] Y.W Liu, C.M. Han, X.Y. Ma, J.H. Yang, X.P. Feng, Y.B. Jiang Tetrahedron Lett. 2018, 59, 650.
[4] C.Y. Chen, X.W. Lu, M.C. Holland, S.C Lv, X.B. Ji, W. Liu, J. Liu, D. Depre, P. Westerduin, Eur. J. Org. Chem., 2020, 548.

