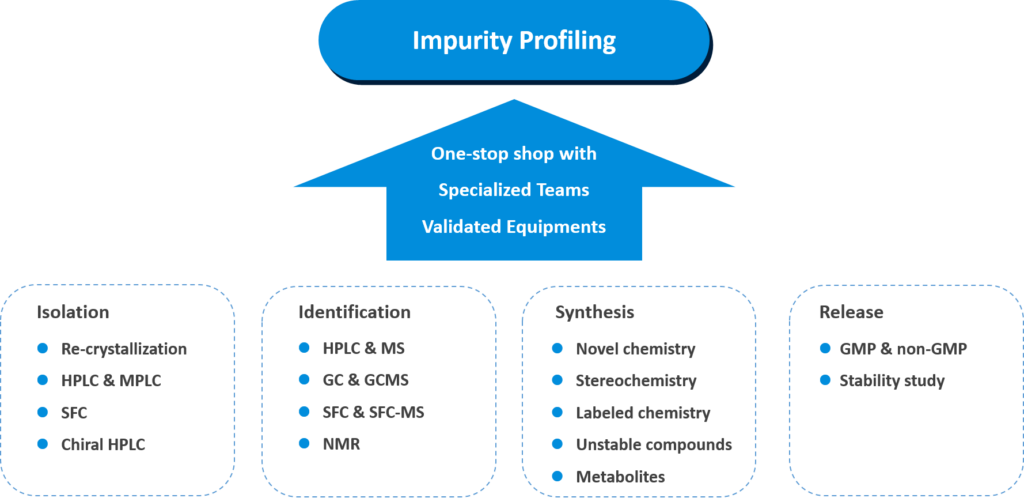
- Provides one-stop shop services of impurity isolation, identification, synthesis and analytic release
- Delivered 500+ impurities of new launched drugs and phase I-III clinic candidates
- Delivered 500+ impurities of generic drugs for local pharmaceutical companies
- Plan to prepare 20000+ impurities of generic drugs in future
- Highly experienced in isomer isolation and formation control, metabolite synthesis, instable impurity synthesis and API degradation
- Dedicated team with 50+ synthetic chemists in the impurity synthesis and 50+ analytical chemists in impurity isolation and GMP/non-GMP analysis