Structure-based optimization of hydroxylactam as potent, cell-active inhibitors of lactate dehydrogenase
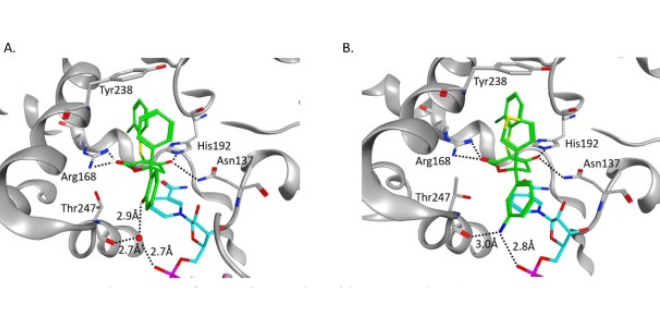
Structure-based design was utilized to optimize 6,6-diaryl substituted dihydropyrone and hydroxylactam to obtain inhibitors of lactate dehydrogenase (LDH) with low nanomolar biochemical and single-digit micromolar cellular potencies. Surprisingly the replacement of a phenyl with a pyridyl moiety in the chemical structure revealed a new binding mode for the inhibitors with subtle conformational change of the LDHA active site. This led to the identification of a potent, cell-active hydroxylactam inhibitor exhibiting an in vivo pharmacokinetic profile suitable for mouse tumor xenograft study.
https://doi.org/10.1016/j.bmcl.2022.128576
Authors
BinQingWeia KirkRobargea Sharada S.Labadiea JinhuaChenb Laura B.Corsona AntonioDiPasqualea Peter S.Dragovicha CharlesEigenbrota MarieEvangelistaa Benjamin P.Faubera AnnaHitza RebeccaHonga Kwong WahLaib WenfengLiub ShuguangMaa ShivaMaleka ThomasO’Briena JodiePanga DavidPetersona LaurentSalphatia DeepakSampatha StevenSiderisa MarkUltscha ZijinXub IvanaYena DongYub QinYuea AiheZhoua Hans E.Purkeya
- a Genentech, Inc., 1 DNA Way, South San Francisco, CA 94080, USA
- b WuXi AppTec, 288 Fute Zhong Road, Waigaoqiao Free Trade Zone, Shanghai, 200131, China
Related Content
Delivering comprehensive end-to-end solutions for creating, identifying, and supporting preclinical candidates, from discovery to development, with our integrated chemistry and...
VIEW RESOURCE
