Progress and Challenges in Tumor Vaccine Development
Introduction:
Cancer is a major public health concern, and this disease is a leading cause of death worldwide. There are over 200 different types of cancer, and such complexity has slowed the rate of progress being made by researchers. Encouragingly, the emergence of immunotherapies, especially tumor vaccines, brings hope to cancer patients. Personalized tumor vaccines (tailored for each patient) have shown promising results in the treatment of solid tumors such as malignant melanoma, lung cancer, and glioma. With continuous development of technologies involving high-throughput sequencing and artificial intelligence, and increasing understanding of tumor immunity research, research and development on tumor vaccines is in full swing. In this article, Dr. Jian Xiang from the Oncology and Immunology Unit of WuXi AppTec shares his experience and case study examples of the progress being made in tumor vaccine development.
Principles and types of cancer vaccines
The tumor microenvironment (TME) is a complex ecosystem, consisting of stromal cells, immune cells, extracellular matrix, and cytokines. Immunotherapy depends on autologous immunity to kill tumor cells and inhibit tumor growth by activating or enhancing the body’s immune system through different cell types.
Cancer immunotherapies include checkpoint inhibitors, tumor vaccines, therapeutic antibodies, and small molecule agonists. These modalities have shown potent antitumor effects in a variety of malignancies. Among these therapeutic approaches, cancer vaccines have become a research hotspot in recent years (Figure 1). For diseases like cancer, it is important that therapeutic vaccines contain adjuvants that promote strong T cell responses. Adjuvants prolong antigen exposure to dendritic cells (DCs) and induce their maturation. Antigen sources include peptides, tumor-associated proteins, autologous tumor lysates, and tumor-derived mRNAs and DNAs. After vaccination, tumor antigens are phagocytosed by DCs and these cells then present antigenic peptides (loaded onto major histocompatibility complex molecules) to cytotoxic T lymphocytes (CTLs) to activate them, eventually resulting in tumor cell death. Neoantigens are ideal targets for therapeutic cancer vaccines because they are produced by genetic mutations in tumor cells and therefore not expressed in healthy tissues.
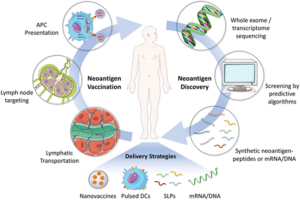
Figure 1. Development of Personalized Cancer Vaccine (Front Immunol, 9:1499. 2018 )
Due to their outstanding performance during the COVID-19 pandemic, mRNA vaccines were ranked first by MIT Technology Review as “Global Breakthrough Technology in 2021”. This recognition has greatly promoted the research and development of mRNA vaccines and created a more engaging environment for the application of mRNA technology in the treatment of multiple diseases. Compared with other types of tumor vaccines (Figure 2), mRNA vaccines can encode all antigens while maintaining a relatively simple production processes.
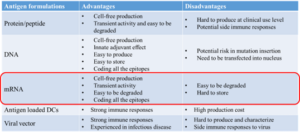
Figure 2. Types of Cancer Vaccines
Progress in the research and development of mRNA cancer vaccines
Structurally, mRNA vaccines are mainly divided into two parts; one part is the designed antigen-encoding mRNA, and the other part is the mRNA delivery system (Figure 3). Because mRNA is a large and negatively charged molecule, it cannot diffuse across the lipid bilayer of the cell membrane. Instead, mRNA molecules will be phagocytosed by innate immune cells and degraded by ribonuclease enzymes. Hence, the delivery vector of mRNA is very important, as suitable carriers can reduce the non-specific degradation of mRNA and deliver it to the target location. At present, mRNA vaccines are grouped into two categories: non-replicating mRNA and self-amplifying mRNA. Commonly used delivery systems include lipid nanoparticles (LNPs), polymeric nanoparticles, and protamines.
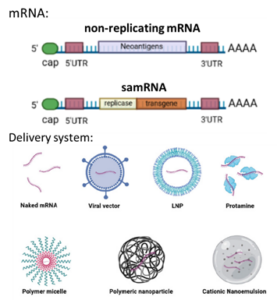
Figure 3. Composition of mRNA (Vaccines 9, 1060, 2021)
Since the global outbreak of COVID-19, two mRNA-based vaccines (Moderna and Pfizer-BioNTech) have received Emergency Use Authorizations from the United States Food and Drug Administration (FDA). At present, therapeutic areas for mRNA vaccines have expanded beyond COVID-19 into cancers and other infectious diseases. According to available clinical trial data (Figure 4), multiple mRNA tumor vaccines are currently undergoing clinical trials.
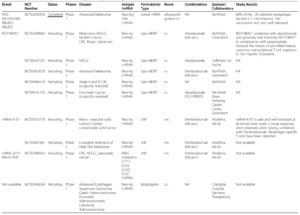
Figure 4. Clinical Trials of Antigen-encoding mRNA Vaccines (Mol Cancer 20, 41, 2021)
Moderna currently has more than 45 product lines, of which 5 are related to tumors. Among them, a personalized tumor vaccine, mRNA-4157, can load up to 34 antigen-encoding mRNA sequences which can be rapidly designed and produced. The disease control rate (DCR) of mRNA-4157 was shown to be 90% in Phase I trials and this vaccine is now undergoing two phase II clinical trials (Figure 5).
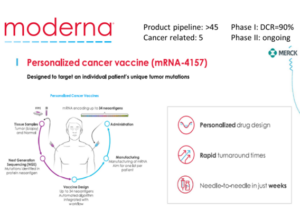
Figure 5. Moderna mRNA-4157 Cancer Vaccine (https://www.modernatx.com/research/product-pipeline)
BioNTech has more than 30 product lines, of which 15 are targeting tumors. The company’s FixVac tumor platform was established to develop tumor vaccines using mRNA encoding common tumor associated antigens (TAAs) from cancer patients. The BNT111 vaccine against advanced melanoma and the BNT113 vaccine against HPV16-positive head and neck cancer have both entered clinical phase II trials. In addition, the personalized tumor vaccine BNT122 against patient-derived specific antigens has also entered clinical phase I and II trials (Figure 6).
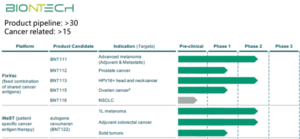
Figure 6. The BioNTech tumor vaccine platform (Fig. https://www.biontech.com)
Research and development challenges and strategies for cancer vaccines
There are challenges in the development of all cancer immunotherapy treatments, and cancer vaccines are no exception. The effectiveness of vaccines has always been a key focus of interest. There are two main causes for dissatisfactory clinical curative effects: (1) Insufficient antigen delivery: DCs are unable to function as effective antigen-presenting cells (APCs) for T cells, or immunosuppressive cells inhibit the activation of T cells; (2) “Cold tumor”: a low number of tumor-specific T cells, suppression of T cell infiltration into TME, and T cell dysfunction/exhaustion. (Figure 7).
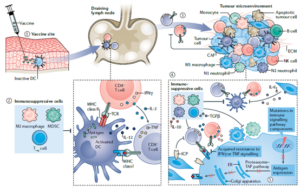
Figure 7. Challenges of Cancer Vaccine Effectiveness (Nat Rev Cancer 21, 360 – 378, 2021)
So how can researchers improve the efficacy of therapeutic cancer vaccines? One common clinical approach is combination therapy, which combines cancer vaccines with other immunotherapies such as immune checkpoint inhibitors, CAR-T, oncolytic viruses, or immuno-stimulants (Figure 8).
The delivery system is also vital for successful cancer vaccine development, especially for mRNA vaccines. The safety of the delivery system must always be considered. Reported side effects of Moderna and Pfizer’s COVID-19 mRNA vaccine are likely related (in part) to their carriers. Therefore, reducing toxic side effects, improving efficiency, enhancing degradability, and simplifying preparation procedures are all future directions for carrier optimization. Due to the poor cellular permeability and stability of some antigens, vaccine development also requires a stable delivery system. The suitable carrier should allow for long-term and heat-tolerant storage of antigens, while ensuring that antigens can be delivered to the APC in the targeted region, thereby providing long-term immunity in vivo. The ability to manipulate the surface chemistry or other properties of nanoparticles enables their targeted delivery to specific tissues and cells (Figure 8).
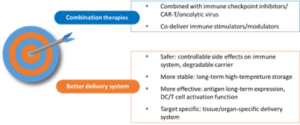
Figure 8. Design Strategy of Cancer Vaccines
Development and biological evaluation of cancer vaccines
Research and development of cancer vaccines typically undergoes four major steps: Step 1. Antigen/neo-antigen identification; Step 2. Formulation preparation; Step 3. Immunogenicity testing; Step 4. Efficacy testing and mechanism-of-action validation toward the tumor model; (Figure 9).
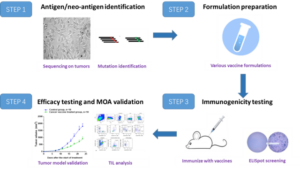
Figure 9. R&D Steps of Cancer Vaccine Development
Case study
Case 1: mRNA Cancer Vaccine
We first developed a mouse melanoma cell line with stable overexpression of ovalbumin (OVA), named B16-OVA. In vitro results showed that OVA peptides were able to be presented to tumor cells. T cells derived from OT-I mice were co-incubated with tumor cells to perform a T cell-mediated tumor cell killing assay. Results indicated that pulsed OVA can be used as a target for antigen-specific T cell killing. Based on this finding, we prepared an OVA-specific mRNA tumor vaccine. ELISpot assay results revealed that a strong immune response was generated in mice after immunization. The results of in vivo experiments showed that the specific immunity induced in mice by this vaccine was capable of warding off a tumor challenge, significantly inhibiting tumor growth and prolonging mouse survival time (Figure 10).
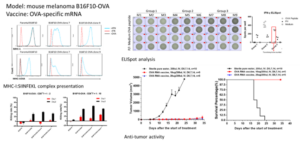
Figure 10. OVA-specific ex vivo and in vivo system to evaluate the effects of mRNA Cancer Vaccine
Cancer vaccines have significant advantages over traditional chemo-/radio- treatments or targeted therapies because vaccines can active immunologic memory and provide long-term protection from tumor recurrence. Tumor re-challenge experiments proved this notion. Mice exhibiting complete tumor regression after vaccination were selected and then injected with tumor cells again. The tumor re-challenge experiment was repeated twice with similar results showing no tumor formation in mice (Figure 11), indicating that long-term immune protection was induced successfully. We also tracked the distribution of mRNA vaccines in mice through IVIS imaging instrument, which provides a method for studying the tissue targeting property of vaccines.
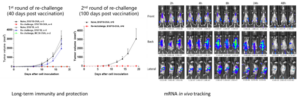
Figure 11. Immune Protection of OVA-specific mRNA Cancer Vaccine and in vivo Tracking of mRNA Vaccine
Case 2: Dendritic Cell Cancer Vaccine
DCs, as professional antigen presenting cells, can be loaded with tumor antigens in vitro. Tumor-antigen-loaded DC leads to induction of tumor-antigen specific T cells and exerts anti-tumor effects after DC cancer vaccine injection. Bone marrow-derived dendritic cells (BMDCs) were isolated, loaded with tumor antigens and matured via LPS treatment, followed by characterization of purity and surface costimulatory molecule expression via flow cytometry. In vivo experiments showed that tumor growth was significantly inhibited after treatment with this DC cancer vaccine. On the top of the results, DC vaccine exhibited stronger anti-tumor effects compared with the simply mixture treatment of agonist and antigen (Figure 12).

Figure 12. Biological Evaluation of DC Cancer Vaccines
WuXi AppTec Oncology and Immunology Platform
The Oncology and Immunology Unit of WuXi AppTec works efficiently with partners through our leading biology research services platform. We cover all of your research needs ranging from target identification and validation, in vitro and in vivo pharmacology discovery and IND enabling studies, and translational research to clinical biomarker testing to accelerate drug discovery for cancer, autoimmune and inflammatory diseases, and rare genetic diseases. Our comprehensive platforms, with a wealth of homologous and humanized murine models, tumor cell lines, and state-of-the-art technology, offer end-to-end solutions for traditional small molecules, antibody drugs, bifunctional molecules, and other new modalities, aiming to provide cost-effective and high-quality services for customers (Figure 13).
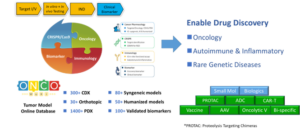
Figure 13. WuXi AppTec Oncology and Immunology Platform
WuXi AppTec | Tumor Models:
- Learn about our extensive collection of tumors model for small & large molecule drug discovery by clicking HERE
- View our comprehensive platform of immuno-oncology services by clicking HERE
- AACR Poster: “Development of an integrated in vitro and in vivo OVA-specific system for cancer vaccine discovery”
Related Content
NK cells are effector innate lymphoid cells (ILCs) that do not require antigen-specific priming. NK cells are not only killer...
VIEW RESOURCEA new wave of drugs that achieves targeted delivery of radioisotopes is rapidly gaining momentum. As the discovery pipeline widens...
VIEW RESOURCE
