OncoWuXi Express: Evaluation of T Cell Engagers
Introduction:
OncoWuXi Express will continue to keep you informed about updates to our online tumor model database (OncoWuXi Database), as well as our recent progress in cancer and autoimmune research. In this issue, we would like to share with you our preclinical pharmacological evaluation platform for T cell engagers.
https://onco.wuxiapptec.com
Introduction
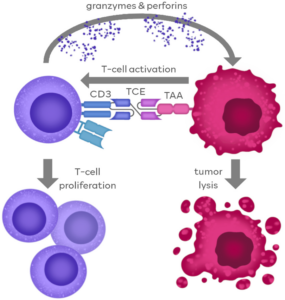
T-cell engagers (TCEs) are a class of bispecific antibodies that can simultaneously target and bind to tumor cells and T cells, thereby activating T cells and mediating their cytotoxic effects on tumor cells [1]. This dual binding mechanism allows T cells to accurately identify and attack tumor cells. TCEs have advantages of high specificity and strong immune response in anti-tumor therapy. Structurally, TCEs bind to tumor cells and T cells via the T-cell recognition region (mainly targeting CD3e) and the tumor-associated antigen (TAA) recognition region, promoting the formation of immune synapses between the two. Activated T cells release perforin and granzymes to kill tumor cells. Additionally, TCEs can boost T cell proliferation, thereby enhancing their cytotoxic effects on the tumor [2].
Figure 1. Action mechanism of TCE drugs [1]
Case Study I: In vitro activity evaluation of TCE
Evaluation of TCE activity using a reporter cell line
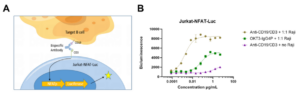
WuXi Biology selected the Jurkat T-cell line and successfully constructed a reporter cell line, Jurkat-NFAT-Luc, for the nuclear factor of activated T-cells (NFAT) signaling pathway (Fig. 2A). This cell line can be used to detect NFAT signals triggered by CD3e activation, thereby assessing the related activity of TCEs. By co-culturing the Jurkat-NFAT-Luc cells with Raji cells (a type of Burkitt’s lymphoma cell highly expressing human CD19 protein) and adding a gradient concentration of CD19/CD3 bispecific antibody, the results showed that the fluorescence intensity was positively correlated with the concentration of the bispecific antibody, and no significant change in fluorescence value was observed in the absence of target cells (Fig. 2B). The TCE activity evaluation method based on reporter cell line not only has low detection cost but also high repeatability.
In addition, the optimization of affinity towards the CD3e end is particularly important for TCE drugs, as excessive affinity may lead to TAA-independent T-cell activation. Reporter cell line can also be used to evaluate the activity of antibodies targeting the CD3e end in the absence of target cells.
Fig. 2. Working Principle and Detection Example of Jurkat-NFAT-Luc
Detection of T-cell cytotoxicity against tumor cells mediated by CD19/CD3 bispecific antibody and cytokine secretion
TCEs can simultaneously recognize T cells and tumor cells, thereby mediating the cytotoxicity of T cells against tumor cells, and influencing the tumor immune microenvironment through cytokine secretion. Therefore, the WuXi Biology team co-incubated peripheral blood mononuclear cells (PBMCs) and tumor cells at a certain ratio and added a gradient of CD19/CD3 bispecific antibody. Cytotoxic activity of the CD19/CD3 bispecific antibody against tumor cells was detected using flow cytometry (Fig. 3A) and the level of cytokine release was measured via ELISA (Fig. 3B).
TCE mediates the cytotoxic effect of T cells on tumor cells and affects the tumor microenvironment, while also promoting the proliferation and activation of T cells. The WuXi Biology team labeled T cells with CFSE and detected the proliferation of T cells through flow cytometry (Figure 3C), and evaluated the TCE-mediated T cell activation by detecting the early activation marker CD69 and late activation marker CD25 (Figure 3D).
Figure 3. In vitro activity evaluation of CD19/CD3 bispecific antibody
Case Study II: In vivo anti-tumor efficacy evaluation of TCE
PBMC-humanized subcutaneous tumor-bearing mouse model
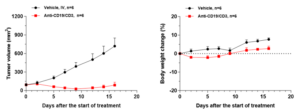
The WuXi Biology team inoculated lymphoma Raji cells subcutaneously in NOG-dKO mice and humanized them by injecting PBMCs through the tail vein. When the tumor size reached 100mm3, the mice were injected with CD19/CD3 bispecific antibody. As shown in Figure 4, the CD19/CD3 bispecific antibody significantly inhibited tumor growth, without any significant effect on the weight of the mice during the administration period.
Figure 4. In vivo activity evaluation of CD19/CD3 bispecific antibody
PBMC-humanized orthotopic tumor-bearing mouse model
Orthotopic tumor models can better simulate the in vivo microenvironment of primary tumors, so evaluation of the distribution and efficacy of TCEs in this model has higher clinical relevance. The WuXi Biology team used the small cell lung cancer cell line SHP-77-Luc to create an orthotopic lung cancer model and injected the mice with DLL3/CD3 bispecific antibody (DLL3 TCE). The activity of DLL3 TCE was evaluated by measuring the fluorescence intensity of the tumor cells. The results show that DLL3 TCE can significantly inhibit the proliferation of tumor cells (Figure 5).
Figure 5. Activity evaluation of DLL3 TCE in the PBMC-humanized orthotopic lung tumor model
Hematopoietic stem cell (HSC) humanized subcutaneous tumor-bearing mouse model
HSCs can differentiate into various immune cells, and compared to the PBMC transplantation model, the HSC transplantation model has a more complete humanized immune cell population, including monocytes and lymphocytes. We validated the activity of claudin18.2/CD3 bispecific antibody (CLDN18.2 TCE) in the HSC-humanized tumor model. As shown in Figure 6, CLDN18.2 TCE can significantly inhibit the growth of hCLDN18.2-Mia PaCa 2 tumors. The analysis of tumor-infiltrating lymphocytes showed that, compared with the solvent control group, the proportion of T cells in the TCE administration group significantly increased, and the expression of the activation marker granzyme B increased, while the proportion of various cells in the peripheral blood did not change significantly. This data shows that this TCE can specifically activate T cells within the tumor, without significant impact on the peripheral system.
Figure 6. Activity evaluation of CLDN18.2 TCE in the HSC-humanized tumor model
Summary
WuXi Biology has established a comprehensive preclinical pharmacology and efficacy evaluation platform to assist in the research and development of T cell engagers.
Reference:
[1] Goebeler, Maria-Elisabeth & Bargou, Ralf. (2020). T cell-engaging therapies — BiTEs and beyond. Nature Reviews Clinical Oncology.
[2] Cech, P.; Skórka, K.; Dziki, L.; Giannopoulos, K. T-Cell Engagers—The Structure and Functional Principle and Application in Hematological Malignancies. Cancers 2024, 16, 1580.
[3] Tapia, Antonio & Álvarez-Vallina, Luis & Sanz, Laura. (2023). Bi- and trispecific immune cell engagers for immunotherapy of hematological malignancies. Journal of Hematology & Oncology.
Technical Contact:
Pharmacology-BD-Translation@wuxiapptec.com
Business contact:
Mahnaz_Arjomand@wuxiapptec.com (US)
dave_madge@wuxiapptec.com (Europe)
fumio_itoh@wuxiapptec.com (Japan)
sycho@wuxiapptec.com (Korea)
xu_longji@wuxiapptec.com (China)
Register for our OncoWuXi Database via the following website or by scanning the QR code to search for more information about oncology and immunology related models.
https://onco.wuxiapptec.com
WuXi AppTec | Oncology Platform:
Related Content
Natural killer (NK) cells play a crucial role in the immune system and can recognize and kill cancer cells. Research...
VIEW RESOURCEResearch on cancer immunotherapy has firmly established immune cells as key players in effective cancer treatment. Peptide vaccines directly targeting...
VIEW RESOURCE