OncoWuXi Express: Efficacy of JAK Inhibitors in a Type II Collagen-induced Arthritis Model
Introduction:
OncoWuXi Express will continue to keep you informed about updates to our online tumor model database (OncoWuXi Database), as well as our recent progress in cancer and autoimmune research. In this issue, we present an evaluation of the efficacy of different JAK subtype inhibitors in a Type II collagen-induced arthritis model, along with corresponding in vivo models of rheumatoid arthritis.
https://onco.wuxiapptec.com
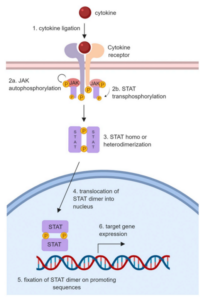
Janus Kinase (JAK) is an important class of signal transduction proteins, composed of four family members: JAK1, JAK2, JAK3, and TYK2. JAK plays a crucial role in the growth, differentiation, and function of immune cells. When activated by cytokines, JAK phosphorylates the intracellular tail of the receptor subunit, creating docking sites for signal transducers and activators of transcription (STAT) proteins. These phosphorylated STAT transcription factors form dimers and migrate into the nucleus, regulating transcription of target genes (Figure 1). JAK inhibitors (JAKi) can obstruct cytokine signal transduction and promote anti-inflammatory and immunomodulatory effects. JAK inhibition as a treatment strategy has gained widespread recognition in the field of autoimmune disease treatment. A variety of JAKi have passed clinical trials and are now in use, including Tofacitinib (a JAK1/3 inhibitor) primarily for rheumatoid arthritis (RA) treatment, and Baricitinib (a JAK1/2 inhibitor) used mainly for treating moderate to severe active RA [1].
Figure 1. JAK-STAT Signaling Pathway [1]
In addition, the JAK1 inhibitor Upadacitinib has been reported to improve the symptoms of RA patients in two Phase II clinical trials and one Phase III clinical study with inadequate response to methotrexate and TNF-α antagonists [2]. These results reveal the potential and prospective clinical efficacy of JAKi in the field of RA treatment. RA, the most prevalent chronic systematic autoimmune disease globally, affects approximately 1% of the worldwide population. The main clinical feature of RA is joint swelling caused by the accumulation of immune cells in the joint synovium, resulting in bone loss, muscle waste, and cartilage damage. JAKi can block inflammatory responses such as factor signaling, synovial inflammation, and autoantibody production by regulating tumor necrosis factor (TNF), interferon, and interleukin within the inflammatory factor signaling pathway, thereby alleviating joint lesions (Figure 2) [3].
Figure 2. Function of JAK-STAT pathway in pathogenesis of RA [2]
To further understand the roles of different JAK inhibitors in the pathogenesis and progression of RA, WuXi Biology’s Platform evaluated five non-selective or selective JAKi, including Tofacitinib (a non-selective JAKi), Upadacitinib (a JAK1 inhibitor), CEP-33779 (a JAK2 inhibitor), Ritlecitinib (a JAK3 inhibitor) and Deucravacitinib (a TYK2 inhibitor).
WuXi Biology used male DBA/1 strain mice as the research objects, inducing arthritis by subcutaneous injection of a CFA emulsion containing Bovine CII at the tail, and monitoring the severity of joint inflammation and the extent of erythema and swelling in the mice’s paws. Mice in each group were evaluated and treated according to their score and body weight when they reached a predetermined threshold. Throughout the dosing period, all mice were scored for joint swelling three times weekly, and their body weights were obtained.
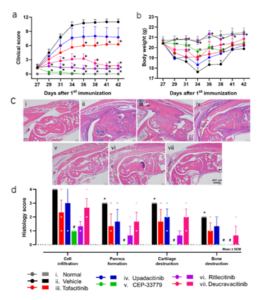
Drawing upon the clinical scores, body weight, and histopathological results of the healthy group, control group, and treatment group, WuXi Biology successfully and stably induced a CIA model with a substantial clinical scoring window. The pathological results showed that the mice displayed the pathological characteristics of synovial hyperplasia, cartilage, and bone destruction. Furthermore, all five JAKi-treated groups demonstrated varying degrees of relief of foot swelling, and pathological histological changes were alleviated (Figure 3).
Figure 3. Evaluation of symptoms and joint injuries in CIA mice. (a) Arthritis clinical score; (b) Body weight of mice in each CIA group; (c) Representative pathological picture of mice paws; (d) Histologic scores of inflammatory cell infiltration, synovial hyperplasia, cartilage destruction, and bone destruction, ranging from 0 (normal) to 4 (severe).
To further comprehend how JAK inhibitors affect the immune system and its potential mechanism of action in RA treatment, WuXi Biology utilized flow cytometry to identify and count T cells, B cells, and myeloid cells in the spleen and their subsets. We analyzed 34 cytokines in plasma using Olink® technology and performed a hierarchical cluster analysis of the results. Compared with the control group, non-selective JAKi, JAK2, JAK3, and TYK2 inhibitors effectively inhibited the differentiation and proliferation of T cells. Non-selective JAKi, JAK1, and JAK2 inhibitors effectively inhibited the differentiation and proliferation of B cells, while non-selective JAKi, JAK1, JAK2, and TYK2 inhibitors resulted in a decrease in the number of myeloid cells (Figure 4a). TYK2 inhibitors significantly inhibit the expression of inflammatory cytokines (Figure 4b).
Figure 4. Detection of immune cell phenotype and plasma protein. (a) Heat map of spleen T cells, B cells, and myeloid cells with their subpopulations; (b) Hierarchical clustering heat map of Olink® in plasma from CIA mice.
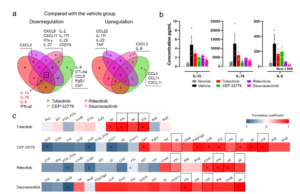
By comparing and correlating the symptoms (clinical scores), pathology, cytokines, and immune cells of CIA mice before and after different JAKi treatments, WuXi Biology delineated the commonalities and unique characteristics of different JAKi in RA treatment. WuXi Biology screened cytokines with a 30% differential between the treatment group and vehicle group in protein expression. These results showed that the plasma concentrations of IL-1β, IL-16, and IL-6 in the treatment group generally trended downwards compared to the control group. To evaluate the correlation between plasma proteins and clinical scores in CIA mice, WuXi Biology found that the downregulation of IL-1β, IL-16, and IL-6 in plasma was highly correlated with the alleviation of arthritis symptoms in CIA mice through Spearman rank correlation analysis (Figure 5). In addition, different JAKi treatments also cause changes in their unique cytokines, suggesting that these cytokines may be an entry point for JAKi to explore the mechanism of RA.
Figure 5. Cytokine screening data for JAKi regulation. (a) Cytokine changes in CIA mice treated with different JAKi compared to the control group; (b) Compared with the control group, the plasma concentrations of IL-1β, IL-16 and IL-6 in each treatment group showed a decreasing trend; (c) Spearman rank correlation analysis of plasma protein and clinical score in CIA mice.
Summary:
This study represents a comprehensive comparison of non-selective or selective JAKi in the CIA mouse model, offering valuable insights into immune modulation and potential implications for optimizing the benefit-risk profile of selective JAKi in the therapy of RA.
References:
[1] Jamilloux Y, El Jammal T, Vuitton L, Gerfaud-Valentin M, Kerever S, Sève P. JAK inhibitors for the treatment of autoimmune and inflammatory diseases. Autoimmun Rev. 2019 Nov;18(11):102390.
[2] Burmester GR, Kremer JM, Van den Bosch F, Kivitz A, Bessette L, Li Y, Zhou Y, Othman AA, Pangan AL, Camp HS. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018 Jun 23;391(10139):2503-2512.
[3] Tanaka Y, Luo Y, O’Shea JJ, Nakayamada S. Janus kinase-targeting therapies in rheumatology: a mechanisms-based approach. Nat Rev Rheumatol. 2022 Mar;18(3):133-145.
[4] Stump KL, Lu LD, Dobrzanski P, Serdikoff C, Gingrich DE, Dugan BJ, Angeles TS, Albom MS, Ator MA, Dorsey BD, Ruggeri BA, Seavey MM. A highly selective, orally active inhibitor of Janus kinase 2, CEP-33779, ablates disease in two mouse models of rheumatoid arthritis. Arthritis Res Ther. 2011 Apr 21;13(2):R68.
Technical Contact:
Pharmacology-BD-Translation@wuxiapptec.com
Business contact:
Mahnaz_Arjomand@wuxiapptec.com (US)
dave_madge@wuxiapptec.com (Europe)
fumio_itoh@wuxiapptec.com (Japan)
sycho@wuxiapptec.com (Korea)
xu_longji@wuxiapptec.com (China)
Register for our OncoWuXi Database via the following website or by scanning the QR code to search for more information about oncology and immunology related models.
https://onco.wuxiapptec.com
WuXi AppTec | Oncology Platform:
Related Content
Introduction Endometriosis is a common gynecological condition that significantly impacts the health of women of reproductive age worldwide. Approximately 10%...
VIEW RESOURCEPrecision-cut tissue slices (PCTS) are an effective ex vivo model for studying human diseases, including those involving the tissue microenvironment....
VIEW RESOURCE