OncoWuXi Express: Efficacy of DLL3 CAR-T in SHP-77 Tumor Models
OncoWuXi Express: Evaluation of DLL3 CAR-T in SHP-77-Luc Tumor Models
Introduction:
OncoWuXi Express will continue to keep you informed about updates to our online tumor model database (OncoWuXi Database), as well as our recent progress in cancer and autoimmune research. In this issue, we showcase our DLL3 CAR-T cell preparation and evaluation of their anti-tumor efficacy.
https://onco.wuxiapptec.com
Small Cell Lung Cancer (SCLC) accounts for approximately 15% of all lung cancer cases and is marked by high malignancy, rapid progression, and poor prognosis, resulting in a median overall survival of only about 13 months. Approximately 70% of patients have metastasized at time of diagnosis [1]. There is a significant unmet medical need for novel therapies with better efficacy, while avoiding off-target toxicity.
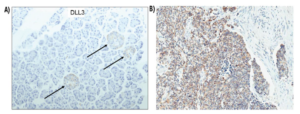
An emerging immunotherapy, chimeric antigen receptor (CAR) T cell therapy, has shown great promise in treating SCLC. Notch pathway, a highly conserved cell signaling pathway, is involved in various developmental processes, including the development of pulmonary neuroendocrine cells. The inhibitory Notch ligand Delta-Like Ligand 3 (DLL3) is a direct transcriptional target of the key neuroendocrine transcription factor, Achaete-Scute Family BHLH Transcription Factor 1 (ASCL1), expressed in about 85% of SCLCs. Notably, DLL3 expression levels are low in normal cells and mainly confined to the Golgi apparatus and cytoplasmic vesicles [2] but are higher in SCLC cells and transported to the cell surface (Figure 1). Therefore, DLL3 is considered a potential therapeutic target for SCLC. The team led by Dr. Renier Brentjens at the Roswell Park Comprehensive Cancer Center developed a chimeric antigen receptor (CAR) against DLL3 and engineered it to secrete interleukin 18 (IL-18). These IL-18-secreting CAR T cells display significant antitumor efficacy in murine models [1].
Figure 1: Expression of DLL3 in original peritumoral tissue (A) and SCLC tumor tissue (B)[2]
WuXi Biology provides in vitro and in vivo pharmacodynamic evaluation services for CAR-T cell therapies targeting different antigens. We have conducted efficacy evaluations in cell line-derived small-cell lung cancer SHP-77-Luc in murine subcutaneous and orthotopic tumor models with DLL3 CAR-T therapy.
Case Study:
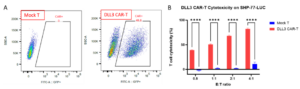
WuXi Biology isolated and activated T cells from commercially available peripheral blood mononuclear cells (PBMCs) and successfully developed DLL3 CAR-T cells using lentiviral transduction technology, with a CAR positive rate of 48.8% as detected by flow cytometry (Figure 2A). In vitro assays showed that DLL3 CAR-T cells had a strong killing effect on SHP-77-Luc cells, which was positively correlated with the effector-to-target (E/T) ratio.
Figure 2: The positive rate of CAR in DLL3 CAR-T (A) and the in vitro cytotoxicity (B)
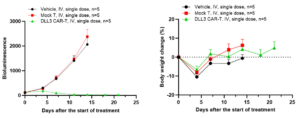
In the SHP-77-Luc subcutaneous tumor model, after tail-vein injections of CAR-T cells, it was demonstrated that DLL3 CAR-T cells had a strong anti-tumor effect with almost complete tumor regression (Figure 3).
Figure 3: Antitumor efficacy of DLL3 CAR-T in the SHP-77-Luc subcutaneous tumor model
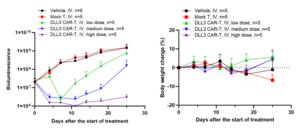
In the SHP-77-Luc orthotopic lung model, different doses of CAR-T cells were injected via the tail vein, and tumor growth was monitored in the IVIS® small animal in vivo optical imaging system. The results showed that DLL3 CAR-T cells exhibited tumor-suppressive activities with a clear dose-dependence effect (Figure 4).
Figure 4: Antitumor efficacy of DLL3 CAR-T in the SHP-77-Luc orthotopic lung model
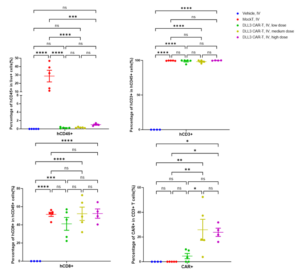
18 days after injecting CAR-T cells into the SHP-77-Luc orthotopic lung model, we collected peripheral blood and utilized flow cytometry to analyze the proportions of T cells and CAR+ cells. The analysis indicated that T cells remained detectable in the mice 18 days post-treatment, with CAR+ cells also identified within the DLL3 CAR-T groups (Figure 5).
Figure 5: The percentage of hCD45+, hCD3+, hCD8+, and CAR+ cells in the peripheral blood of mice in the orthotopic lung model after 18 days of treatment
Conclusion
Compared to subcutaneous tumor model, orthotopic model can simulate the development and metastasis of tumor better. Meanwhile, due to the characteristic of CAR-T bio-distribution in vivo [3], using orthotopic model to evaluate CAR-T drugs can reflect the efficacy of CAR-T therapy more accurately. Additionally, because some of the organs, such as lung and brain, are more sensitive to inflammation, the orthotopic model, compared to the subcutaneous model, can expose the potential toxicity of CAR-T drug better, providing more guidance for the clinical research.
References
[1] Jaspers JE, Khan JF, Godfrey WD, et al. IL-18-secreting CAR T cells targeting DLL3 are highly effective in small cell lung cancer models. J Clin Invest. 2023 May 1;133(9):e166028.
[2] Rudin CM, Reck M, Johnson ML, et al. Emerging therapies targeting the delta-like ligand 3 (DLL3) in small cell lung cancer. J Hematol Oncol. 2023 Jun 24;16(1):66.
[3] Qiong Wu, Yan Wang, Xinyu Wang, et al. Pharmacokinetic and pharmacodynamic studies of CD19 CAR T cell in human leukaemic xenograft models with dual‐modality imaging. J Cell Mol Med. 2021 Aug; 25(15): 7451–7461.
Technical Contact:
Pharmacology-BD-Translation@wuxiapptec.com
Business Contacts:
xu_longji@wuxiapptec.com (China)
Mahnaz_Arjomand@wuxiapptec.com (US)
dave_madge@wuxiapptec.com (Europe)
sycho@wuxiapptec.com (Korea)
fumio_itoh@wuxiapptec.com (Japan)
Register for our OncoWuXi Database via the following website or by scanning the QR code to search for more information about oncology and immunology related models.
https://onco.wuxiapptec.com
WuXi AppTec | Oncology Platform:
Related Content
In addition to identifying chemical starting points for early drug discovery processes, DNA-encoded library (DEL) technology has now expanded to...
VIEW RESOURCEThe efficacy of CAR-T therapy is substantially influenced by the tumor microenvironment. This is particularly evident when PD-L1 is activated...
VIEW RESOURCE