Evaluation of the efficacy of ADC in vitro and in vivo
Abstract
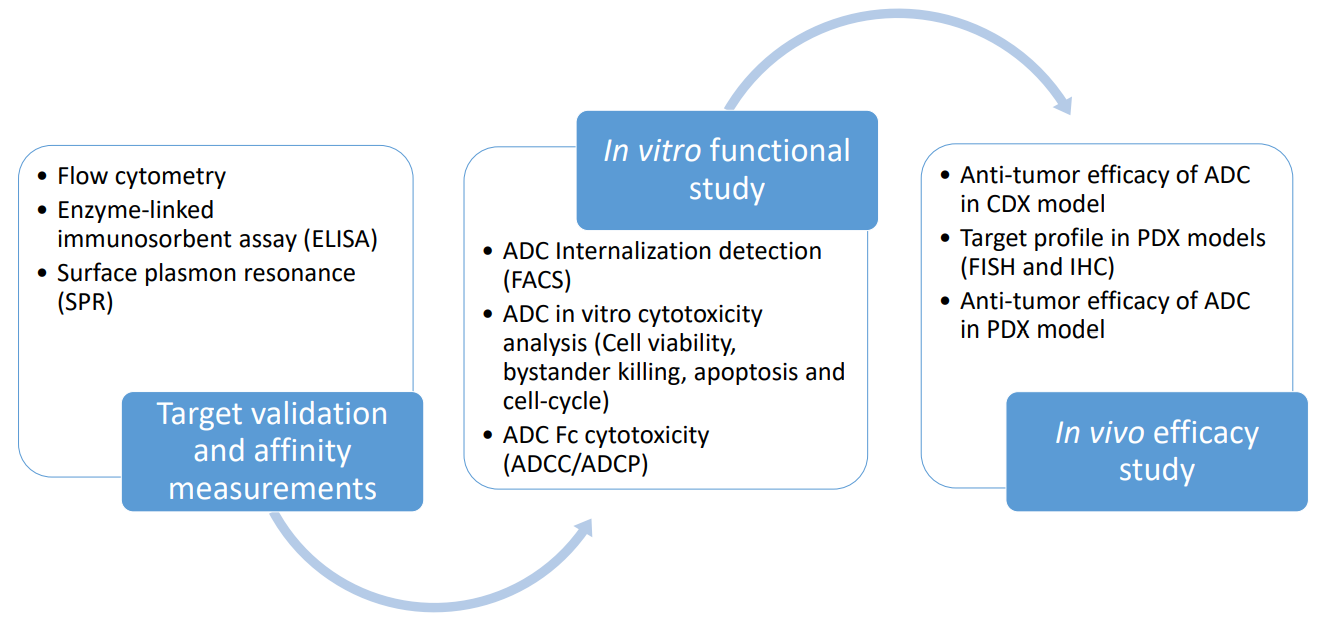
Antibody drug conjugates (ADCs) are promising complexes with therapeutic potential that aimed to the treatment of solid tumor and hematological malignancies. Compared with the therapeutic monoclonal antibody, an ADC-derived monoclonal antibody is conjugated with cytotoxic agents (payloads) which can deliver potent cellular toxins to targeted cancer cells specifically. We have established a state-of-the-art platform to support the evaluation of the efficacy of ADCs, or the combination strategy of ADCs and other anti-cancer therapies for pre-clinical drug discovery (including target validation, efficient internalization, cytotoxic effects and in vivo efficacy testing).
In order to explore the mechanism of action and the functional effects of ADCs, our flow cytometry platform (BD LSRFortessa X20) supports target validation and the process of internalization in a quick, reliable and reproducible way. Also, we are able to construct customized antigen-specific overexpression or KI cells used for the development of ADCs which target on specific antigens. In terms of in vivo ADCs assessment, our abundant CDX and PDX tumor model platform facilitates the process of in vivo ADC evaluation, and we are able to assess the efficacy of ADC (or the combination treatment of ADC and other anti-tumor drugs) in several animal models.

10 ADC_5566_Jun Huang
Related Content
Antibody-targeted therapies—including antibody-drug conjugates (ADCs), antibody-oligonucleotide conjugates (AOCs), and degrader-antibody conjugates (DACs)—are reshaping precision medicine by enabling highly selective delivery...
VIEW RESOURCEAntibody-drug conjugates (ADCs) hold great promise as targeted cancer therapeutics, but their complex structure poses significant development and safety challenges....
VIEW RESOURCE
