NHP Pain Animal Models
Introduction:
OncoWuXi Express will continue to keep you informed about updates to our online pharmacology model database (OncoWuXi Database), as well as our recent progress in preclinical research. In this issue, we showcase non-human primate pain animal models.
https://onco.wuxiapptec.com
Pain is defined as an unpleasant sensory and emotional experience associated or similar to actual or potential tissue damage. According to relevant statistics, there have been more than 300 million patients with chronic pain in China, and pain has become the third major health challenge after cardiovascular and cerebrovascular diseases and tumors [1]. Pain is divided into acute pain and chronic pain. Acute pain acts as a defense mechanism capable of counteracting noxious stimuli, infection, homeostatic dysfunction in the body, and secondary injury. Chronic pain, on the other hand, covers many types, including migraine, musculoskeletal pain, osteoarthritis, diabetic neuropathy, and cancer-related chronic pain. Existing analgesic drugs, such as opioids and nonsteroidal anti-inflammatory drugs (NSAIDs), although they have some effect in analgesia, have significant side effects and abuse risks. Therefore, the development of analgesic drugs focused on new targets undoubtedly has a broad market prospect and important clinical significance.
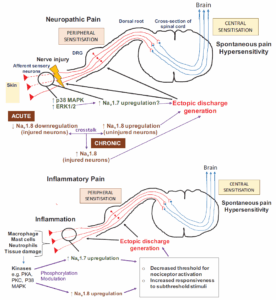
Sodium channels have received increasing attention in the research and development of new analgesic drugs. Voltage-dependent sodium channels underlie electrical signaling in all neurons. There are nine different types of sodium channels, encoded by different genes. Among them, three sodium channels, Nav1.1, Nav1.2, and Nav1.6, are widely expressed in the central nervous system (CNS) and peripheral nervous system (PNS), while Nav1.7, Nav1.8, and Nav1.9 are mainly expressed in some of the peripheral neurons [2, 3] (Figure 1). Notably, the loss of Nav1.7 channel function makes Nav1.7 congenitally painless, which is a major candidate target for the development of new analgesic drugs [4].
Among the potent small-molecule inhibitors of Nav1.7 discovered to date, sulfonamide compounds have demonstrated particularly outstanding performance. These compounds exhibit high selectivity for Nav1.7 channels, with even greater selectivity for human Nav1.7 compared to Nav1.7 channels from other species. Currently, Xenon Pharmaceuticals is advancing a preclinical development pipeline targeting Nav1.7[ 5 ] .
Additionally, drug development targeting Nav1.8 has become another important focus. On January 30, 2025, the FDA approved Vertex Pharmaceuticals’ Journavx (suzetrigine) for the treatment of moderate-to-severe acute pain in adults. This marked the first approved Nav1.8-targeted inhibitor and represented a major breakthrough in the field of non-opioid analgesics [6]. Further validating the significant market potential of this target in pain management, Eli Lilly announced on May 27, 2025, the acquisition of SiteOne Therapeutics’ Nav1.8 inhibitor pain pipeline, which is poised to enter Phase II clinical trials [7].
Fig. 1. Pain Mechanisms Regulated by Nav1.7 and Nav1.8 [ 3 ]
During the development of Nav1.8 small molecule inhibitors, Merck & Co., Inc. discovered that although these molecules could selectively inhibit human Nav1.8 channels in vitro, they exhibited a significant decrease in potency when targeting rodent Nav1.8 channels. This characteristic somewhat limits the use of rodent models in in vivo screening for this class of drugs [8]. Cynomolgus monkeys (Macaca fascicularis), as non-human primates, share high genetic similarity with humans, making them particularly advantageous for in vivo screening of such drugs.
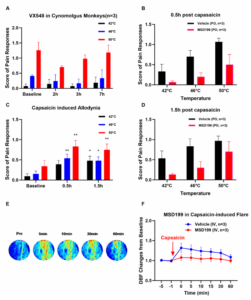
WuXi Biology has established and validated a series of pain animal models in cynomolgus monkeys. These models primarily include thermal pain models, capsaicin-induced hyperalgesia models, and capsaicin-induced flare models. In the thermal pain model, the WuXi AppTec team utilized a Quantitative Sensory Testing (QST) device commonly used in clinical settings to apply different temperature stimuli to the animals and evaluate their pain responses. Experimental results showed that the Nav1.8 inhibitor VX-548 effectively suppressed the animals’ thermal pain response 2 hours after oral administration (Figure 2A).
In the capsaicin-induced hyperalgesia model (Figure 2C), the Nav1.8 inhibitor MSD199 effectively reduced capsaicin-induced hyperalgesia after oral administration (Figures 2B and 2D). Finally, in the capsaicin-induced flare model, the team utilized a laser speckle imaging system to monitor changes in skin blood flow (flare response) following intradermal injection of capsaicin (Figure 2E). By comparing flare responses within the same animal across different tests, the team found that the flare response demonstrated good stability and reproducibility. Furthermore, MSD199 was shown to effectively reduce the capsaicin-induced flare response following intradermal injection (Figure 2F).
Figure 2. Effects of Nav1.8 Inhibitors in Pain Models
The above results indicate that targeting sodium ion channels can significantly suppress pain responses in animals. This series of validated pain models not only provides an efficient and reliable experimental platform for preclinical drug screening studies but also enables researchers to evaluate the efficacy of potential therapeutic agents. As a result, this approach offers a scientific basis for efficacy assessment in the early stages of new drug development, thereby accelerating the drug development process.
References:
[1] Fan blue hair. China Pain Medicine Development Report. Tsinghua University Press, 2020.
[2] Yekkirala AS, Roberson DP, Bean BP, Woolf CJ. Breaking barriers to novel analgesic drug development. Nat Rev Drug Discov. 2017 Aug; 16 (8): 545-564. doi: 10.1038/nrd. 2017.87.
[3] Hameed S. Nav1.7 and Nav1.8: Role in the pathophysiology of pain. Mol Pain. 2019 Jan-Dec; 15:1744806919858801. doi: 10.1177/1744806919858801.
[4] Drissi I, Woods WA, Woods CG. Understanding the genetic basis of congenital insensitivity to pain. Br Med Bull. 2020 May 15; 133 (1): 65-78. doi: 10.1093/bmb/ldaa003.
[5] XENON. Product Pipeline. From: https://www.xenon-pharma.com/product-pipeline/.
[6] Vertex Pharmaceuticals Inc. Vertex Announces FDA Approval of JOURNAVX ™ (suzetrigine), a First-in-Class Treatment for Acute Adults with Moderate-to-Severe Pain. (2025). From: https://news.vrtx.com/news-releases/news-release-details/vertex-announces-fda-approval-journavxtm-suzetrigine-first-class.
[7] Eli Lilly. Lilly to expand its pain pipeline with acquisition of SiteOne Therapeutics. (202 5). From: https://www.prnewswire.com/news-releases/lilly-to-expand-its-pain-pipeline-with-acquisition-of-siteone-therapeutics-302465735.html
[8] McDevitt DS, Vardigan JD, Zhou X, Rosahl TW, Zhou H, Price EA, Clements MK, Li Y, Varghese N, Krasowska-Zoladek A, Stachel SJ, Breslin MJ, Burgey CS, Kraus RL, Pall PS, Henze DA, Santarelli VP. Humanized NaV1.8 rats overcome cross-species potency shifts in developing novel NaV1.8 inhibitors. Neurobiol Pain. 2025 Mar 6; 18:100182. doi: 10.1016/j.ynpai. 2025.100182.
For further information, please contact us by:
Pharmacology-BD-Translation@wuxiapptec.com
Scan the QR code below to register with our OncoWuXi database for free access to more pharmacological model data and information.
https://onco.wuxiapptec.com
WuXi AppTec | Pain Platform:
- Pain models; click HERE to view our models and measurements
Related Content
Introduction: OncoWuXi Express will continue to keep you informed about updates to our online pharmacology model database (OncoWuXi Database), as...
VIEW RESOURCEEpilepsy is a chronic brain disorder characterized by recurring seizures, which are episodes of uncontrolled and abnormal electrical activity in...
VIEW RESOURCE