Innovative R&D Strategies for Peptide Drugs in Obesity Treatment
Introduction:
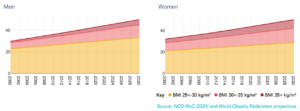
Obesity has emerged as a pressing global public health issue, with its prevalence escalating at an unprecedented rate across the world. Body Mass Index (BMI) is a commonly used indicator to assess the degree of obesity. According to the World Obesity Atlas 2025, by 2030, over 2.9 billion adults globally will have a high BMI (BMI > 25 kg/m²), of whom 1.1 billion will be classified as obese (BMI ≥ 30 kg/m²) [1] (Figure 1). This epidemic is particularly concerning due to its strong association with a spectrum of chronic diseases, including type 2 diabetes, hypertension, coronary heart disease, stroke, and various forms of cancer.
Figure 1. Percentages of men and women (aged 20+) living with high BMI, (2000-2030) [1]
In recent years, the approval of novel peptide-based drugs such as semaglutide and tirzepatide has marked a breakthrough in obesity management [2,3]. These drugs work by mimicking the action of endogenous peptides—semaglutide targets the glucagon-like peptide-1 (GLP-1) receptor, while tirzepatide activates both the GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) receptors. Both agents significantly reduce body weight and simultaneously improve metabolic parameters, including blood glucose, lipids, and blood pressure, thereby lowering cardiovascular risk. This has addressed a critical unmet need in obesity treatments and offers patients a more effective therapeutic option.
The successful application of peptide drugs in the treatment of obesity has attracted considerable attention in drug development. As innovative peptide drug strategies emerge, precise and effective preclinical pharmacological evaluation methods are crucial for advancing peptide drug research.
Multi-Target Combination Strategies and Preclinical Efficacy Evaluation:
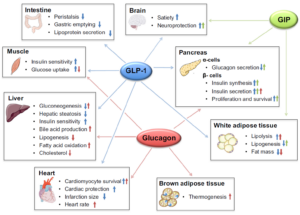
In the rapidly evolving field of anti-obesity drug development, a growing trend is emerging toward multi-targeted therapies. By engaging multiple key signaling pathways simultaneously, more pronounced weight loss effects can potentially be achieved. The three primary targets currently being explored involve GLP-1, GIP, and glucagon (GCG). Agonism at the GCG receptor, in particular, is thought to play a significant role in weight reduction. GCG primarily acts on the liver to promote glycogenolysis and gluconeogenesis, increasing glucose output into the bloodstream. Concurrently, GCG receptor agonism can modulate overall energy metabolism, enhancing energy expenditure and potentially optimizing substrate utilization, which may help prevent excess energy from being stored as fat. This regulatory effect makes targeting the GCG receptor pathway a component of interest in anti-obesity drug combinations (Figure 2).
Figure 2. Effects of GLP-1 (blue arrows), glucagon (red arrows), and GIP (green arrows) on energy metabolism in key metabolic tissues. Small arrows in boxes pointing upwards indicate an increase or improvement of the respective metabolic function, while arrows pointing downwards indicate a decrease. [4]
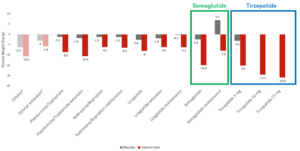
Clinical trials have shown that multi-target combinations offer advantages in weight loss efficacy. For instance, tirzepatide, a dual GLP-1/GIP receptor agonist, demonstrates superior weight loss compared to the single GLP-1 receptor agonist semaglutide (Figure 3). This suggests that by targeting multiple metabolic pathways, such drugs can more effectively regulate appetite, energy intake, and energy expenditure, leading to more effective weight loss [5].
Figure 3. Mean percent (%) weight change reported in the main phase 3 and extension trials of the FDA-approved anti-obesity medications. [5]
However, a key challenge in preclinical research is determining whether the efficacy of multi-target drugs can be accurately modeled using standard rodent models. For example, semaglutide, a GLP-1 receptor agonist, demonstrates robust efficacy in both humans and preclinical diet-induced obesity (DIO) mouse models (over 80%), largely due to the high homology of the GLP-1 receptor between humans and mice. In contrast, multi-target drugs like tirzepatide, which activate both GIP and GLP-1 receptors for synergistic effects, present complexities. In human studies, the efficacy of tirzepatide appears to rely on activity at both receptors. However, in conventional DIO mouse models, blocking GLP-1 receptor activity abolishes tirzepatide’s efficacy, while blocking GIP receptor activity reportedly has minimal impact on its effects on insulin secretion or blood glucose. This discrepancy suggests that in conventional DIO mice, the drug’s effects may primarily stem from GLP-1 receptor activation. The lower amino acid sequence homology of the GIP receptor (around 80%) between humans and mice may account for this difference. GIP receptor-mediated pathways in mice may not fully replicate the human pharmacological response, hindering the demonstration of multi-target synergy (Figure 4).
Figure 4. Efficacy Evaluation of Multi-Target GLP-1 Based Drugs in Obesity Treatment
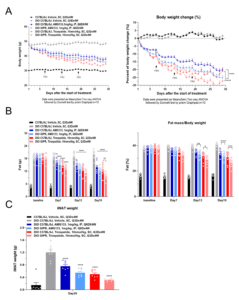
To overcome this limitation, the Metabolic Disease team at WuXi Biology developed humanized DIO mouse models expressing the human GIP receptor. These models were used to evaluate the efficacy of dual-target drugs, including tirzepatide and AMG133 (a GLP-1 receptor agonist combined with a GIP receptor antagonist), compared with their effects in conventional DIO mice [6]. Both agents showed enhanced weight loss in the humanized models (Figure 5A). Additional body fat analysis confirmed a greater reduction in fat percentage in these mice, consistent with their observed weight loss (Figure 5B). Examination of terminal tissue samples further showed that humanized models more accurately reflected the synergistic activity on white and brown adipose tissue, validating their utility in preclinical evaluation (Figure 5C). These findings demonstrate that humanized models offer more translatable data for evaluating the efficacy of multi-target peptide therapies.
Figure 5. Efficacy Evaluation of Dual-Receptor GLP-1 Based Drugs in DIO Humanized Mice
“Muscle-Preserving Weight Loss” Strategies and Preclinical Efficacy Evaluation
Another key consideration in anti-obesity drug development is the preservation of muscle mass. Maintaining lean body mass during weight reduction is a growing focus, and numerous strategies are being explored to address this challenge.
At the 2024 American Diabetes Association (ADA) conference, early research demonstrated that monotherapies and combination therapies may help mitigate muscle loss during weight reduction, paving the way for drug development [7-9]. Building on this, WuXi AppTec researchers investigated the combination of semaglutide with an apelin receptor (APJ) agonist currently in Phase III trials [10]. The results showed that this combination significantly enhanced fat loss while preserving muscle mass. Although there was no significant change in absolute muscle mass, the overall weight loss resulted in an increased proportion of lean body mass relative to total body mass. This indicates that combination therapy can promote fat loss while minimizing muscle depletion (Figure 6).
Figure 6. Efficacy Evaluation of Semaglutide Combined with APJ Agonist in Mouse Models
To assess whether the preserved muscle was functional, the team conducted muscle function and behavioral assays, including the wire hang test, a standard measure of muscle strength and endurance in rodents. The results confirmed that the combination therapy not only preserved muscle mass but also ensured the functionality of the preserved muscle, which is in line with the concept of “muscle-preserving weight loss” (Figure 7).
Figure 7. Effect of Semaglutide Combined with APJ Agonist on Muscle Function in Mice
Traditional behavioral tests, however, can be influenced by inter-individual variability and operator-dependent factors, leading to data inconsistency. Additionally, evaluating the impact of anti-obesity drugs on muscle function is challenging, as physical activity levels tend to decrease in obese individuals.
To overcome these limitations, WuXi Biology employed a more refined and objective approach using electrophysiological platforms to monitor changes in muscle fiber characteristics and quantify muscle stress. This method provides a more comprehensive and accurate assessment of muscle function and drug efficacy.
Sustained Weight Loss Strategies and Preclinical Efficacy Evaluation
Another critical focus in anti-obesity drug development is ensuring sustained weight loss. Some anti-obesity drugs lead to rapid weight regain after discontinuation, compromising their effectiveness and potentially causing adverse health effects.
To address this, preclinical models that simulate the time course of weight regain after drug withdrawal are essential. WuXi Biology conducted weight regain studies in DIO mice following established treatment protocols. After a defined treatment period, drug administration was discontinued, and the mice were observed for 3 to 4 weeks. The appetite-suppressing effects of the drug diminished quickly after withdrawal, with some animals even exceeding the control group’s food intake, exacerbating the weight regain (Figure 8).
Figure 8. Efficacy Evaluation of Sustained Weight Loss Treatment in Mouse Models
Notably, the composition of the regained weight was primarily fat mass (Figure 9), contrasting with the weight loss phase, where both muscle and fat are typically reduced. This pattern of fat-predominant rebound not only reduces the net benefit of therapy but may also worsen metabolic outcomes. These findings underscore the importance of developing peptide drugs that can deliver sustained weight loss and minimize rebound after cessation of treatment.
Figure 9. Significant Increase in Fat Mass and Changes in Lean Body Mass in DIO Mice After Discontinuation of Tirzepatide Treatment
A Final Word
Peptide drugs show considerable promise in the treatment of obesity. Through approaches such as multi-target combinations, muscle-preserving and sustained weight loss, researchers continuously optimize both the mechanisms of action and preclinical evaluation frameworks. Well-designed preclinical pharmacological models are essential in this process, providing valuable insights into the mechanisms of energy balance and supporting the discovery of new therapeutic targets, thereby laying a solid foundation for subsequent clinical applications of peptide drugs.
References
- World Obesity Atlas 2025
- Mahapatra MK, Karuppasamy M, Sahoo BM. Semaglutide, a glucagon like peptide-1 receptor agonist with cardiovascular benefits for management of type 2 diabetes. Rev Endocr Metab Disord. 2022 Jun;23(3):521-539.
- https://tirzepatide.lilly.com/
- J. Brandt, T. D. Müller, R. D. DiMarchi, M. H. Tschöp, K. Stemmer, Peptide-based multi-agonists: a new paradigm in metabolic pharmacology. Journal of Internal Medicine. 2018 Dec:581-602
- Chakhtoura, M., Haber, R., Ghezzawi, M., Rhayem, C., Tcheroyan, R., & Mantzoros, C. S. (2023). Pharmacotherapy of obesity: an update on the available medications and drugs under investigation. EClinicalMedicine, 58, 101882.
- Véniant, M.M., Lu, SC., Atangan, L. et al. A GIPR antagonist conjugated to GLP-1 analogues promotes weight loss with improved metabolic parameters in preclinical and phase 1 settings. Nat Metab 6, 290–303 (2024).
- Dinko Gonzalez Trotter, Stephen Donahue, Chris Wynne, Shazia Ali, L. et al. 34-OR: The Effect of Combined Activin A and Myostatin Blockade on Body Composition—A Phase 1 Trial. Diabetes 14 June 2024; 73 (Supplement_1): 34–OR.
- Clifford Bechtold, Ansarullah, Christopher Brynczka, Volkan Granit. et al.2053-LB: Taldefgrobep Alfa Improves Body Composition as Monotherapy and in Combination with Semaglutide in a DIO Mouse Model. Diabetes 14 June 2024; 73 (Supplement_1): 2053–LB.
- Gauthier Schang, Mathilde De Molliens, Emilie Brûlé.et al. 298-OR: Precision-Engineered Activin and GDF Ligand Trap HS235—A Novel Lean Mass–Preserving Treatment for Obesity. Diabetes 14 June 2024; 73 (Supplement_1): 298–OR.
- Winkle, P., Goldsmith, S., Koren, M. J., Lepage, S., Hellawell, J., Trivedi, A.et al. (2023). A First-in-Human Study of AMG 986, a Novel Apelin Receptor Agonist, in Healthy Subjects and Heart Failure Patients. Cardiovascular drugs and therapy, 37(4), 743–755.
Related Content
The global obesity epidemic is a significant public health concern, increasing the risk of various chronic diseases, including diabetes, cardiovascular...
VIEW RESOURCEObesity is a significant risk factor for developing conditions like type 2 diabetes, heart disease, stroke, and several types of...
VIEW RESOURCE