Innovative Animal Models for MASH Drug Development

Integrating drug targets, molecular types, and clinical translation
Introduction:
Metabolic dysfunction-associated steatohepatitis (MASH), a growing global health concern, has garnered increasing attention due to its complex pathological mechanisms, large patient population, and the current lack of effective clinical treatment options. These factors have positioned drug development for MASH as a cutting-edge focus in medical research. This article will review the latest advancements in MASH drug development, focusing on innovations in animal models driven by drug targets and novel molecular entities, as well as key challenges in clinical translation.
Understanding MASH: Disease Overview and Market Potential
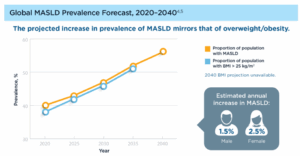
Metabolic dysfunction-associated steatohepatitis (MASH), previously known as nonalcoholic steatohepatitis (NASH), represents the advanced stage of metabolic dysfunction-associated steatotic liver disease (MASLD). Epidemiological studies reveal that MASLD affects over a quarter of the global population, and by 2030, approximately 350 million people worldwide are expected to be living with MASH. The impact of MASH extends far beyond liver pathology, influencing metabolic, cardiovascular, gastrointestinal, endocrine, immune, and renal systems. MASH is strongly linked to metabolic disorders such as obesity and type 2 diabetes and may further lead to cardiovascular diseases. On one hand, from the commercial perspective, the opportunity for MASH drug development is immense. Market projections estimate that the global market for MASH therapies could reach $48.3 billion by 2035 [1]. On the other hand, compounding its complexity, MASH progresses through multiple stages, each characterized by distinct pathological features. This presents considerable challenges for the development of effective preclinical animal models.
Figure 1. Global prevalence projections of MASLD and progression of MASLD pathogenesis [1]
Target-Driven Innovation: The Core of MASH Drug Development
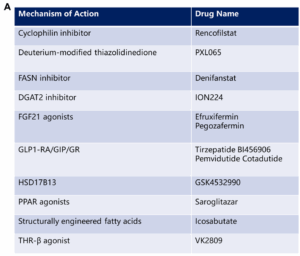
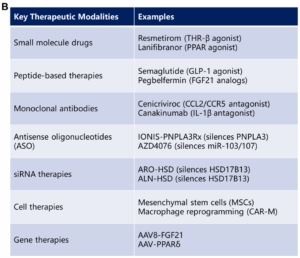
The complex pathological mechanisms of MASH involve multiple metabolic abnormalities and liver pathological changes, making the selection of drug targets a critical aspect of R&D. Currently, there is a diverse range of drug targets associated with MASH, including GLP-1, THR-β, FXR, ASK1, PPAR, and FGF21. These targets can be broadly categorized into two groups: one focuses on metabolic abnormalities, aiming to improve overall metabolic conditions; the other addresses liver pathological changes, with a focus on alleviating liver damage [2-4].
Figure 2. Categories of MASH drugs: A. MASH drugs categorized by mechanism of action; B. MASH drugs categorized by modalities [2, 3]
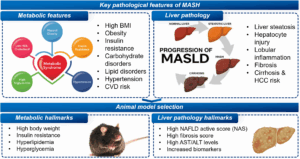
In target-driven innovation, the selection of animal models is crucial. An ideal MASH animal model should simultaneously reflect both metabolic characteristics and liver pathological features. However, there is currently no perfect model that can fully replicate the complexity of human MASH (Figure 3).
Figure 3. Key pathological features of MASH and selection criteria of animal models
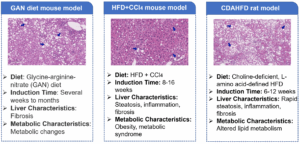
Common representative animal models include the Gubra Amylin (GAN) diet model, the high-fat diet (HFD) combined with carbon tetrachloride (CCl4)-induced mouse model, and the choline-deficient high-fat diet-induced model. However, these models each have limitations in their applications. For instance, the HFD+CCl4 model lacks a comprehensive metabolic abnormality background, whereas the GAN diet model requires an excessively long induction period (Figure 4) [5].
Figure 4. Commonly used MASH animal models [5]
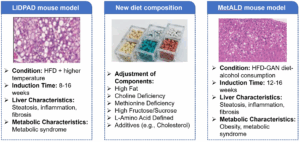
To address these limitations, new models are actively being explored. For instance, modifications to feed composition and animal housing conditions (such as increasing housing temperature) are being investigated to accelerate disease progression and better replicate the pathological processes of metabolic-associated fatty liver disease. Furthermore, the development of metabolic dysfunction-associated alcoholic liver disease (MetALD) model has emerged as a key research focus. By introducing alcohol into the animal model generation process, researchers can more accurately evaluate the efficacy of drugs on alcohol-related MASH (Figure 5).
Figure 5. Novel MASH animal models.
Innovative Approaches: Challenges and Opportunities for New Molecular Entity Drugs
With advancements in drug development technologies, new molecular entity drugs (such as peptides and small nucleic acids) have shown immense potential in the treatment of MASH [6, 7]. The design logic of these drugs is centered on enhancing specificity to target human-specific drug targets precisely. However, this presents new challenges for preclinical research, particularly in how to simulate human targets in animal models to validate drug efficacy.
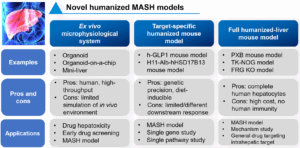
To address these challenges, various innovative models and technologies have been developed. For example, ex vivo microphysiological systems (such as organoids and organ-on-chip technologies) offer high-throughput drug screening platforms. However, their ability to fully replicate in vivo environments remains limited. Additionally, humanized mouse models targeting specific drug targets and fully humanized liver mouse models have been developed to study functional changes in individual genes or pathways, thereby facilitating the evaluation of liver-specific drug targets (Figure 6).
Figure 6. Novel humanized MASH models
Clinical Translation: Reverse Driving from Clinical Needs to Preclinical Research
The ultimate goal of preclinical research is to provide accurate data to support clinical studies and improve clinical translation success rates. However, new discoveries and demands from clinical research, in turn, drive innovation in preclinical studies. In 2023, the term Non-alcoholic Steatohepatitis (NASH) was officially renamed Metabolic Dysfunction-Associated Steatohepatitis (MASH). This change underscores the central role of metabolic factors in the disease and incorporates alcohol consumption into the disease consideration. This conceptual update in clinical understanding has prompted researchers to reevaluate traditional MASH animal models and develop models that reflect the new disease definition, such as models for MetALD.
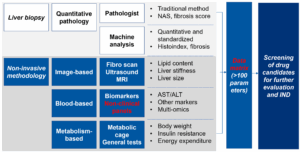
In addition, advancements in clinical diagnostic methods have provided new ideas for preclinical research. While liver biopsy remains the gold standard for MASH diagnosis, its invasive nature limits its application. In recent years, non-invasive diagnostic methods based on hematology, imaging, and multi-omics technologies have emerged. These new methods not only improve diagnostic accuracy and safety but also offer richer endpoints for preclinical research. For instance, incorporating imaging technologies, metabolic phenotyping, and quantitative pathology into preclinical studies can help construct a more comprehensive data matrix, allowing for more precise evaluation of drug efficacy (Figure 7) [2,3].
Figure 7. Traditional to novel preclinical diagnostic methods for MASH
Conclusion and Outlook
MASH drug development is currently in a phase of rapid growth, propelled by innovations in drug targets, new molecular entity drugs, and multidimensional advancements driven by clinical research. Researchers are striving to improve the accuracy of preclinical studies and enhance clinical translation rates by developing more precise animal models, integrating humanized technologies, and adopting non-invasive diagnostic methods. Looking ahead, with a deeper understanding of MASH pathological mechanisms and the continuous emergence of new technologies, there is excellent potential to develop more effective therapeutic strategies to improve the prognosis of hundreds of millions of patients worldwide.
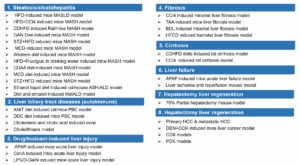
Interdisciplinary collaboration will be essential in this endeavor. The integration of pharmacology, pathology, metabolomics, imaging, and other disciplines will offer robust support for MASH drug research and development. Furthermore, the close linkage between clinical and preclinical studies will accelerate drug development, bringing greater hope to patients. WuXi AppTec’s Biology Service Platform not only provides a variety of MASH animal models but also offers a comprehensive one-stop platform for MASH research (Figure 8). Supporting over 20 global MASH drug submissions, with multiple compounds already advancing into clinical trials, the platform is poised to continue driving innovation and breakthroughs in MASH drug development in the future.
Figure 8. WuXi AppTec’s Liver Disease Platform.
References
- Cellular and Molecular Life Sciences, 2023.
- Souza, Matheus et al. Hepatology (Baltimore, Md.), 10.1097/HEP.0000000000001254. 4 Feb. 2025.
- Caddeo, Andrea, and Stefano Romeo. Clinical and Molecular Hepatology, Vol. 31, Suppl. (2025): S76-S93.
- Harrison, Stephen A et al. Journal of hepatology, S0168-8278(25)00069-8. 10 Feb. 2025.
- Newsome, Philip N, and Rohit Loomba. The Journal of Clinical Investigation, vol. 135,13 e186425. 1 Jul. 2025.
- Newsome, Philip N, and Phil Ambery. “Incretins (GLP-1 receptor agonists and dual/triple agonists) and the liver.” Journal of hepatology vol. 79,6 (2023): 1557-1565.
- Armisen, Javier et al. “AZD2693, a PNPLA3 antisense oligonucleotide, for the treatment of MASH in 148M homozygous participants: Two randomized phase I trials.” Journal of hepatology vol. 83,1 (2025): 31-42.
Related Content
Multi-target drugs targeting GLP1R/GIPR/GCGR have demonstrated superior efficacy for weight loss and glycemic control compared to single-target GLP1R drugs. However,...
VIEW RESOURCEPeptide-based therapeutics have emerged as a significant class of drugs in the pharmaceutical landscape, offering numerous advantages, including high specificity,...
VIEW RESOURCE