DLL3‑related Tumor Models in Humanized Mice

OncoWuXi Express will continue to keep you informed about updates to our online pharmacology model database (OncoWuXi Database), as well as our recent progress in preclinical research. In this issue, we showcase DLL3‑related Tumor Models in Humanized Mice.
Introduction:
The rapid development of tumor immunotherapy has brought new hope to cancer patients. Immune checkpoint inhibitors, adoptive immune cell therapies, and cancer vaccines have all made significant clinical progress [1]. However, these therapies show substantial interindividual variability in efficacy, and only a subset of patients derive durable benefits, so new immunotherapeutic strategies are still needed. The infiltration and functional state of immune cells within the tumor microenvironment not only govern tumor progression and immune evasion but also directly affect treatment response [2].
In recent years, driven by advances in tumor immunology research and breakthroughs in antibody engineering, T cell engagers (TCEs) – a class of emerging immunotherapies that directly recruit and activate T cells to kill tumors – have garnered widespread attention and demonstrated unique clinical potential [3].
DLL3 (delta‑like ligand 3) is a Notch‑pathway–associated inhibitory ligand that is highly expressed in small cell lung cancer (SCLC) but is nearly absent in normal tissues, making it a highly promising therapeutic target. Current DLL3‑targeted strategies mainly comprise antibody‑drug conjugates (ADCs) and bispecific TCEs [4]. TCEs bind both DLL3 on tumor cells and CD3 on T cells to form an “immune synapse” within the tumor microenvironment, directly recruiting T cells to the tumor and mediating specific tumor cell killing. This mechanism can overcome conventional immune‑evasion pathways and offers new therapeutic hope for patients with refractory SCLC [5].
Results:
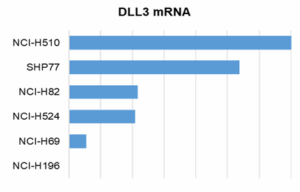
WuXi Biology has established and validated a panel of DLL3‑related humanized mouse models of SCLC. We selected six SCLC cell lines with varying DLL3 expression levels and verified DLL3 protein expression on the cell surface and binding capability to the DLL3/CD3 bispecific antibody by flow cytometry (Figure 1).
Figure 1. DLL3 expression levels and antibody‑binding capacity of SCLC cell lines
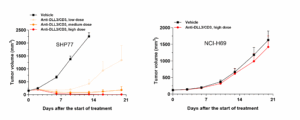
To evaluate in vivo anti-tumor effects involving a human immune system, we first established DLL3‑related tumors in severely immunodeficient mice and concurrently reconstituted a human immune system, creating DLL3‑related SCLC models in humanized mice. Results showed that the anti-tumor efficacy of the DLL3/CD3 bispecific antibody correlated positively with DLL3 protein expression on the tumor cell surface (Figure 2).
Figure 2. Anti-tumor efficacy of the DLL3/CD3 bispecific antibody in PBMC‑humanized SHP77 and NCI‑H69 tumor models
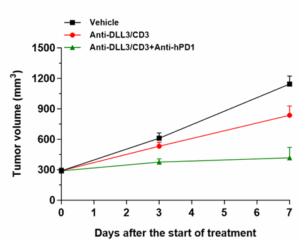
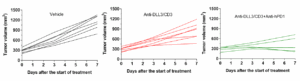
Next, in the PBMC‑humanized SHP77 (with high DLL3 expression) tumor model, we further evaluated the efficacy of combination therapies. The results showed that combining the DLL3/CD3 bispecific antibody with the immune checkpoint inhibitor anti-human programmed cell death protein 1 antibody (anti‑hPD‑1) significantly enhanced anti-tumor efficacy (Figure 3).
Figure 3. Anti-tumor efficacy of the DLL3/CD3 bispecific antibody combined with anti‑hPD‑1 in the PBMC‑humanized SHP77 tumor model
WuXi Biology has established multiple DLL3‑related SCLC models in humanized mice (Table 1) to support our partners’ research. In addition to DLL3, we have successfully validated humanized tumor models for other targets, including CD19, CD20, EpCAM, CEA, CLDN18.2, PSMA, BCMA, PD‑1, and PD‑L1.
Table 1. DLL3‑related humanized SCLC models in WuXi Biology
| Model name | Humanization type |
| SHP77 | PBMC |
| SHP77-luc | PBMC |
| NCI-H524 | PBMC |
| NCI-H82 | PBMC |
| NCI-H69 | PBMC |
| LU-01-1388 | HSC |
References:
[1] Yuanyuan, Zhang., Zemin, Zhang. (2020). The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol, 17(8), 807-821. doi:10.1038/s41423-020-0488-6
[2] Rui, Rui., Liqun, Zhou., Shiming, He. (2023). Cancer immunotherapies: advances and bottlenecks. Front Immunol, 14(0), 1212476. doi:10.3389/fimmu.2023.1212476
[3] Haolong, Lin., Tao, Wang., Jia, Wei. (2025). Strategic innovations: Tackling challenges of immunotherapy in acute myeloid leukemia. Chin J Cancer Res, 37(4), 490-504. doi:10.21147/j.issn.1000-9604.2025.04.02
[4] Charles M, Rudin., Martin, Reck., Melissa L, Johnson(2023). Emerging therapies targeting the delta-like ligand 3 (DLL3) in small cell lung cancer. J Hematol Oncol, 16(1), 66. doi:10.1186/s13045-023-01464-y
[5] Michael J, Giffin., Keegan, Cooke., Edward K, Lobenhofer (2020). AMG 757, a Half-Life Extended, DLL3-Targeted Bispecific T-Cell Engager, Shows High Potency and Sensitivity in Preclinical Models of Small-Cell Lung Cancer. Clin Cancer Res, 27(5), 1526-1537. doi:10.1158/1078-0432. CCR-20-2845
If you have any questions or need further information, please contact us at:
Business Consulting:
Technical Contact:
Pharmacology-BD-Translation@wuxiapptec.com
Scan the QR code below to register at our OncoWuXi database for free.
For more pharmacological model data, information, and consultation.
Related Content
Strategies to stimulate dendritic cell (DC) activity, such as ex vivo generation and priming of DC vaccines, have been explored...
VIEW RESOURCEKRAS mutations act as primary initiators of carcinogenesis in several major cancers and are present in approximately 20 - 25%...
VIEW RESOURCE