Evolution of AAV Therapy: Challenges, Breakthroughs, and Future Directions

Overview of AAV Gene Therapy: Evolution and Current Landscape
Adeno-associated virus (AAV) is a non-enveloped, replication-defective single-stranded DNA virus belonging to the family Parvoviridae and genus Dependoparvovirus. Capable of mediating long-term transgene expression and not associated with any known human diseases, AAV has emerged as a highly attractive therapeutic vector. In 2012, Glybera, the world’s first AAV-based gene therapy, was approved in the European Union, marking a historic transition from bench to bedside [1], though its later withdrawal underscored commercial hurdles. Fueled by rapid technological progress, the field has accelerated. As of 2025, 10 AAV therapies have been approved globally, targeting rare genetic disorders, hematologic diseases, ocular diseases, and other conditions. Notably, Zolgensma, for the treatment of children under 2 years old with spinal muscular atrophy (SMA), generated approximately $1.351 billion in sales in 2021 and has consistently exceeded $1 billion annually since. Additionally, Itvisma (The active ingredient in Itvisma is identical to Zolgensma but formulated at a different concentration) has been approved for the treatment of children, adolescents, and adults aged 2 years and older with SMA carrying the SMN1 gene mutation, demonstrating significant commercial success and validating the therapeutic potential of AAV-based gene therapy [2&11].
Since their emergence in 2012, AAV-based therapies have made significant strides, driven by rapid technological advancements and accelerating industrialization. However, several limitations remain unresolved, including:
- Suboptimal transduction efficiency in certain difficult-to-target cell types
- Broad tissue distribution, leading to off-target transduction and reduced specificity
- Susceptibility to neutralization by pre-existing antibodies
- Limited packaging capacity (~4.7kb), restricting application for diseases requiring large genes.
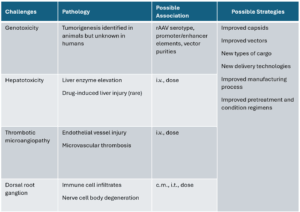
As clinical trials expand and real-world evidence accumulates, safety concerns have emerged, particularly regarding dose-dependent hepatotoxicity, thrombotic events, and potential neurotoxicity observed in some high-dose systemic trials (Figure 1). Overcoming immune barriers, enhancing cell-type/tissue specificity to enable lower, safer doses, and improving manufacturing scalability and yield to reduce costs present challenges for the next generation of AAV vectors.
Figure 1. Challenges and solutions of AAV therapy [3]
AAV Capsid Engineering: Strategies and Applications
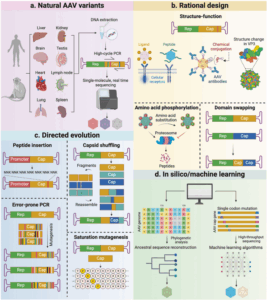
The AAV capsid is a highly symmetrical structure that plays pivotal roles in the viral life cycle—from host cell attachment to nuclear genome delivery. It not only encapsulates and protects the viral genome but also mediates critical biological processes, including host cell receptor binding, intracellular trafficking, genome release, and immune evasion. These multifunctional properties highlight the importance of capsid architecture in virology research and position it as a cornerstone for versatile delivery vectors. To address the limitations of natural serotypes—such as pre-existing immunity, inefficient transduction in target tissues, and off-target effects—researchers are actively developing engineered novel variants with enhanced performance. Currently, three major capsid engineering strategies have been reported: directed evolution, rational design, and hybrid computational-aided design/evolution approaches (Figure 2) [3].
Figure 2. Methods for AAV capsid engineering [3]
Rational Design
Rational design leverages structural and functional insights of AAV capsids to precisely engineer AAV capsids for specific functions-redirecting tropism, enhancing tissue targeting, or evading immune responses through site-directed mutations, peptide insertions, or chemical modifications. Unlike library screening-based approaches, this method imposes minimal selection pressure and is ideal for hypothesis-driven refinements, such as:
- Enhanced Blood-Brain Barrier Penetration: Inserting cell-penetrating peptides (CPPs) into the VR-VIII region of VP3 (e.g., AAV.CPP.16) boosts brain transduction >10-fold in non-human primates [4].
- Improved Tissue Tropism: Triple mutation (F129L/Y445F/Y731F) in AAV6.2FF enhances lung transduction efficiency by 10× vs. wild-type [5].
- Immune Evasion: Mutating heparin sulfate proteoglycan (HSPG) binding residues (R471A or N587A) in AAV2 reduces neutralization by pre-existing antibodies [6].
Directed Evolution
Directed evolution mimics natural selection by generating diverse AAV variant libraries and screening for optimized capsids under selective pressures. It is one of the most widely used AAV capsid engineering strategies. Common library construction methods include: error-prone PCR, DNA shuffling, random peptide insertion, and saturation mutagenesis [7, 8].
De Novo/Computational Bioengineering
Cutting-edge computational tools are revolutionizing AAV engineering. Leveraging big data analysis and complex sequence pattern recognition, scientists can decode hidden structural motifs in AAV sequences, facilitating rational capsid design while reducing screening burdens. Current de novo strategies include:
- Ancestral reconstruction: resurrecting evolutionary precursors (e.g., Anc80) to create vectors with enhanced stability and transduction breadth.
- Algorithm-driven modeling: training neural networks on capsid fitness landscapes to predict functional mutations to engineer superior AAV variants [9, 10].
Synergistic AAV Engineering Strategies
While all three approaches aim to enhance vector performance, their methodologies, applicability, and potential differ significantly [3]. Future advancements will likely integrate computational predictions with rational design, validated by experimental benchmarks (Figure 2). By minimizing data bias, refining machine learning models with high-quality datasets, and iteratively incorporating experimental findings, this synergistic strategy could overcome current limitations in AAV gene therapy, ushering in an era of precision engineering.
Concluding Remarks:
WuXi Biology offers end-to-end AAV delivery solutions, spanning capsid design, promoter optimization, transgene design, vector construction, in vivo/in vitro validation, and clinical sample analysis. Supported by our expert molecular biology team, viral vector platform, comprehensive animal models, and clinical sample analysis support unit, we provide integrated services—from vector design and preclinical evaluation to IND-enabling studies—accelerating the development and translation of AAV-based therapeutics (Figure 3).
Figure 3. Overview of WuXi Biology’s AAV platform services
References
- Issa, Shaimardanova, Solovyeva, Rizvanov, Various AAV Serotypes and Their Applications in Gene Therapy: An Overview. Cells 2023, 12, 785.
- https://bydrug.pharmcube.com/news/detail/d4f0ec4bbac6ee4ede5392a5b24421bb
- Wang, J.-H., Gessler, D. J., Zhan, W., Gallagher, T. L. & Gao, G. Adeno-associated virus as a delivery vector for gene therapy of human diseases. Signal Transduct Target Ther 9, 78 (2024).
- Yao, Y. et al. Variants of the adeno-associated virus serotype 9 with enhanced penetration of the blood-brain barrier in rodents and primates. Nat Biomed Eng 6, 1257–1271 (2022).
- van Lieshout, L. P. et al. A Novel Triple-Mutant AAV6 Capsid Induces Rapid and Potent Transgene Expression in the Muscle and Respiratory Tract of Mice. Mol Ther Methods Clin Dev 9, 323–329 (2018).
- Michelfelder, S. & Trepel, M. Adeno-associated viral vectors and their redirection to cell-type specific receptors. Adv Genet 67, 29–60 (2009).
- Tabebordbar, M. et al. Directed evolution of a family of AAV capsid variants enabling potent muscle-directed gene delivery across species. Cell 184, 4919–4938.e22 (2021).
- Chan, K. Y. et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci 20, 1172–1179 (2017).
- https://bydrug.pharmcube.com/news/detail/d11b11cb79ba14268d809f7521cb8e4f
- Eid, F.-E. et al. Systematic multi-trait AAV capsid engineering for efficient gene delivery. Nat Commun 15, 6602 (2024).
- https://www.fda.gov/news-events/press-announcements/fda-approves-gene-therapy-treatment-spinal-muscular-atrophy

White Paper_Streamlining Gene Therapy with AAVs
Related Content
Gene therapies are enabling a rapid expansion in the scope of treatable disease. Adeno-associated viruses (AAVs) are particularly advantageous delivery...
VIEW RESOURCEAdvances in the understanding of rare disease biology, coupled with innovative technology and therapeutic platforms, has led to progress in...
VIEW RESOURCE