Stepwise Multiple Halogenation Coupling Reaction Strategies
QM Magic Class | Chapter 6
< Magical Power of Quantum Mechanics
In Chapter 5, we introduced “Sequential Cross Coupling Reactions of Polyhalogenated Heterocycles” to design synthetic routes. In this article, we will discuss the use of a complimentary strategy – Stepwise Multiple Halogenation and Coupling Reactions!
Synthesis of Polysubstituted Aromatic Compounds
Polysubstituted aromatic targets are very common targets in organic synthesis. In general, a functional group can be introduced by halogenation and then metal-catalyzed cross-coupling reaction. The resultant intermediates could be treated with a few cycles of halogenation and coupling reaction to provide the final products. However, regio-selectivity of halogenation on complex aromatic substrates is sensitive to the nature and position of the array of their substituents, making it difficult to predict their outcomes accurately, rendering these syntheses “trial and error” processes. In our laboratories, incorporation of QM analyses in planning of these synthetic sequences have proven to be invaluable.
Retrosynthetic Analysis
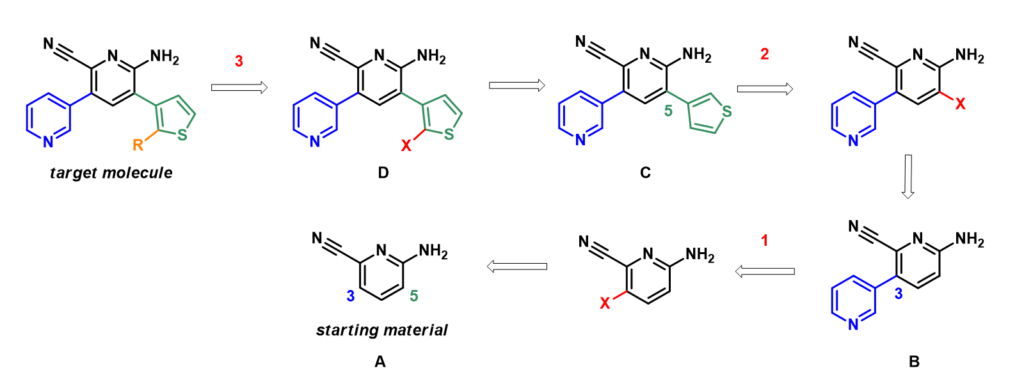
Shown on Figure 1 is the retrosynthetic analysis of the target molecule. Starting with compound A, we need three halogenations and two cross-coupling reactions to obtain the highly functionalized target molecule. Will each halogenation reaction occur in the required position?
HOMO and 13C NMR Calculation
We discussed on Chapter 2 the use of HOMO and calculated 13C NMR to predict the selectivity of electrophilic aromatic substitutions [1]. These parameters are calculated for starting material A, intermediates B and C to assess their chemical properties and feasibility of the proposed synthetic sequence.
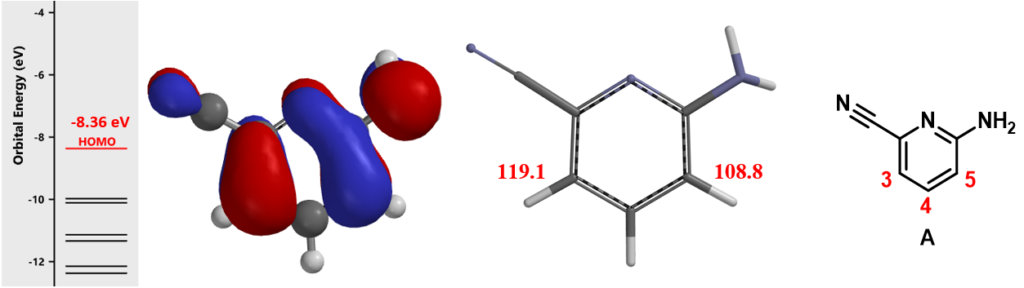
First, how does the cyano and amine groups affect regioselectivity in bromination of pyridine A? With HOMO (Figure 2, left), the 4-position has almost no HOMO lobe distribution, while the HOMO lobes at C-3 and C-5 are similar in size. Calculated 13C NMR chemical shift value is 119.1 ppm at C-3 and 108.8 ppm at C-5, (Figure 2, middle), suggesting that C-5 will be preferentially halogenated, different from what we need. To overcome this intrinsic preference, we planned to use n-Bu4NBr3, which is known to selectively brominate para to amino groups, for reagent control[2]. Being the first step of the synthetic route, this level of uncertainty is acceptable.
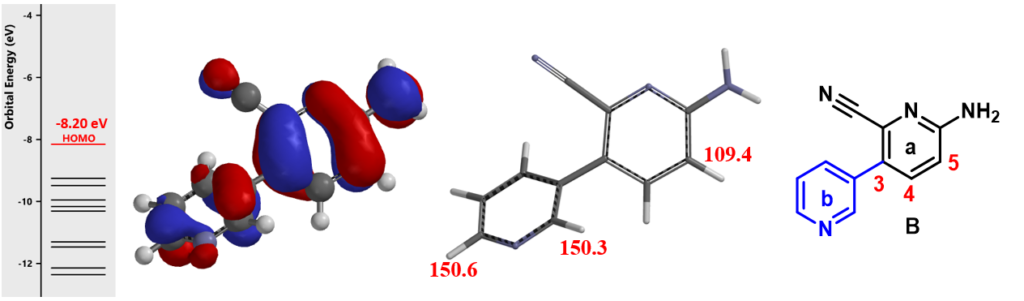
Intermediate B contains two pyridine rings, which one is more nucleophilic? The same QM analysis was carried out. HOMO lobe at the C-5 of the pyridine a ring is the largest among all the substitutable carbons (Figure 3, left), and its calculated chemical shift is 109.4 ppm, lower than those calculated for the other two carbons on the pyridine b ring (Figure 3, middle). These suggest that halogenation of B will occur selectively at C-5.
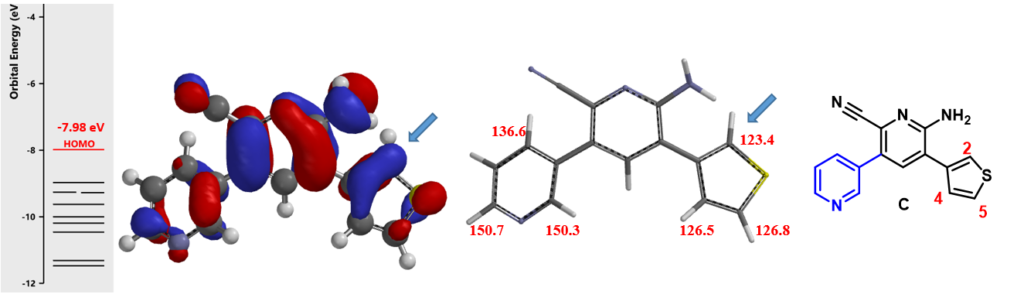
For the third halogenation, an increasingly more complicate intermediate C, QM calculations show that C-2 of thiophene has the largest HOMO lobe (Figure 4, left) and a chemical shift value of 123.4 ppm, the lowest among all substitutable carbons with significant HOMO lobes (Figure 4, middle). Together they enable us to predict with confidence highly selective C-2 halogenation of the thiophene ring.
Next is to find out how well this sequence works in the labs.
Experimental Results Align with QM Calculations
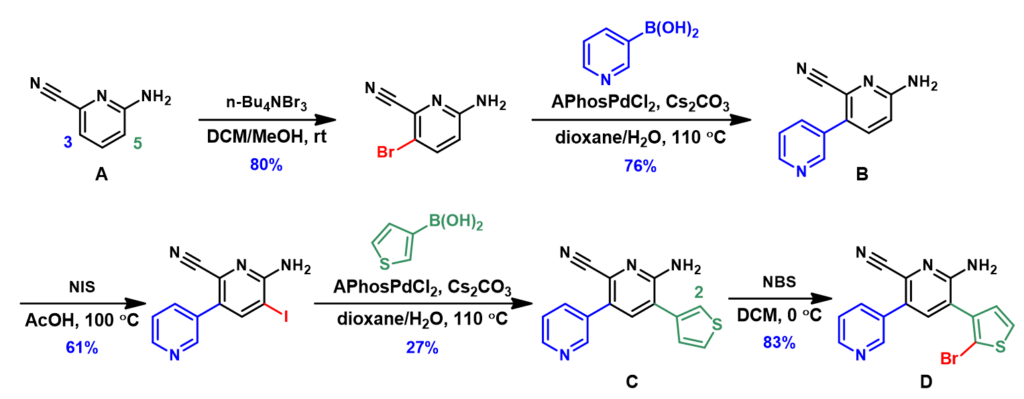
The experimental results of this synthetic route is shown in Figure 5. Reaction of the starting material A with n-Bu4NBr3 indeed allowed us to overcome the intrinsic preference for reaction at C-5, and provided us selectively the required C-3 para bromination product [2]. It is remarkable that QM analysis alerted us to seek for a para selective reagent for the reaction.
The following iodination of intermediate B with NIS and bromination of intermediate C with NBS proceeded selectively as calculated QM data suggested. Stitching these cycles of halogenation and cross coupling reactions in the proper order provided the key bromo intermediate D for further elaborations.
In summary, this chapter introduces the use of stepwise multiple halogenation and coupling strategies to design synthetic routes for target molecules. Success of this strategy hinges on generating the key mono-halo intermediates selectively in each cycle. While in Chapter 5, we obtained the target through the selectively C-X coupling of multi-halogenated substrates, requiring us to know in each reactions which C-X bond is more reactive toward oxidative addition.
Our examples highlighted the use of QM to understand and utilize the inherent chemical properties of starting materials, intermediates, and reagents, to evaluate feasibilities and risks associated with diverse synthetic strategies conceived, to learn chemistry in a more fundamental manner, at a deeper level.
This article is written and edited by Dong Pan, Guqin Shi, Qiuyue Wang, Tommy Lai, John S. Wai.
References:
[1] Spartan’18 Tutorial and User’s Guide (2019). Irvine, CA, USA: Wavefunction, Inc.
[2] n-Bu4NBr3: a) Macsári, I.; Paulsen, K. and Rakos, L. etc. Compounds and Their Use for Treatment of Amyloid Beta-Related Diseases [P]: US, 2012/122843, 2012, A1, 50. b) Berthelot, J.; Guette, C.; Essayegh, M.; Desbene, P. L. and Basselier, J. J. Synth. Commun. 1986, 16, 1641. c) 陈中峻.周克瑜.四丁基三溴化铵在有机合成中的应用.大学化学. 1995, 10, 10.

